
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs.
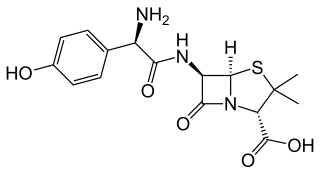
Amoxicillin is an antibiotic medication belonging to the aminopenicillin class of the penicillin family. The drug is used to treat bacterial infections such as middle ear infection, strep throat, pneumonia, skin infections, odontogenic infections, and urinary tract infections. It is taken orally, or less commonly by either intramuscular injection or by an IV bolus injection, which is a relatively quick intravenous injection lasting from a couple of seconds to a few minutes.

Penicillins are a group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for various bacterial infections, though many types of bacteria have developed resistance following extensive use.

Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history.

A medication is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.

Sulfonamide is a functional group that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides.

The rifamycins are a group of antibiotics that are synthesized either naturally by the bacterium Amycolatopsis rifamycinica or artificially. They are a subclass of the larger family of ansamycins. Rifamycins are particularly effective against mycobacteria, and are therefore used to treat tuberculosis, leprosy, and mycobacterium avium complex (MAC) infections.

Rifampicin, also known as rifampin, is an ansamycin antibiotic used to treat several types of bacterial infections, including tuberculosis (TB), Mycobacterium avium complex, leprosy, and Legionnaires' disease. It is almost always used together with other antibiotics with two notable exceptions: when given as a "preferred treatment that is strongly recommended" for latent TB infection; and when used as post-exposure prophylaxis to prevent Haemophilus influenzae type b and meningococcal disease in people who have been exposed to those bacteria. Before treating a person for a long period of time, measurements of liver enzymes and blood counts are recommended. Rifampicin may be given either by mouth or intravenously.

Teicoplanin is an semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall peptidoglycan synthesis. It is used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis.

Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, sold under the brand name Augmentin, among others, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.

Nitrofurazone is an antimicrobial organic compound belonging to the nitrofuran class. It is most commonly used as a topical antibiotic ointment. It is effective against gram-positive bacteria, gram-negative bacteria, and can be used in the treatment of trypanosomiasis. Its use in medicine has become less frequent, as safer and more effective products have become available. Nitrofurazone is listed under California Prop 65, and has demonstrated clear evidence to be mutagenic and carcinogenic during animal studies, and has been discontinued for human use in the USA. The substance is pale yellow and crystalline. It was once widely used as an antibiotic for livestock.

A medication is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.
Multiple drug resistance (MDR), multidrug resistance or multiresistance is antimicrobial resistance shown by a species of microorganism to at least one antimicrobial drug in three or more antimicrobial categories. Antimicrobial categories are classifications of antimicrobial agents based on their mode of action and specific to target organisms. The MDR types most threatening to public health are MDR bacteria that resist multiple antibiotics; other types include MDR viruses, parasites.

Furazolidone is a nitrofuran antibacterial agent and monoamine oxidase inhibitor (MAOI). It is marketed by Roberts Laboratories under the brand name Furoxone and by GlaxoSmithKline as Dependal-M.
Ampicillin/sulbactam is a fixed-dose combination medication of the common penicillin-derived antibiotic ampicillin and sulbactam, an inhibitor of bacterial beta-lactamase. Two different forms of the drug exist. The first, developed in 1987 and marketed in the United States under the brand name Unasyn, generic only outside the United States, is an intravenous antibiotic. The second, an oral form called sultamicillin, is marketed under the brand name Ampictam outside the United States, and generic only in the United States. Ampicillin/sulbactam is used to treat infections caused by bacteria resistant to beta-lactam antibiotics. Sulbactam blocks the enzyme which breaks down ampicillin and thereby allows ampicillin to attack and kill the bacteria.

Doripenem is an antibiotic drug in the carbapenem class. It is a beta-lactam antibiotic drug able to kill Pseudomonas aeruginosa.

Phenazepam is a benzodiazepine drug, first developed in the Soviet Union in 1975, and now produced in Russia and several other countries.
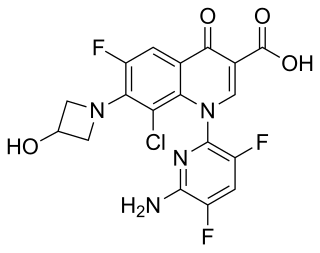
Delafloxacin sold under the brand name Baxdela among others, is a fluoroquinolone antibiotic used to treat acute bacterial skin and skin structure infections.

Quinolone antibiotics constitute a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production.
Antimicrobials destroy bacteria, viruses, fungi, algae, and other microbes. The cells of bacteria (prokaryotes), such as salmonella, differ from those of higher-level organisms (eukaryotes), such as fish. Antibiotics are chemicals designed to either kill or inhibit the growth of pathogenic bacteria while exploiting the differences between prokaryotes and eukaryotes in order to make them relatively harmless in higher-level organisms. Antibiotics are constructed to act in one of three ways: by disrupting cell membranes of bacteria, by impeding DNA or protein synthesis, or by hampering the activity of certain enzymes unique to bacteria.




















