| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,3,5,7-Tetraazaadamantane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| 2018 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.642 | ||
| EC Number |
| ||
| E number | E239 (preservatives) | ||
| 26964 | |||
| KEGG | |||
| MeSH | Methenamine | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1328 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C6H12N4 | |||
| Molar mass | 140.186 g/mol | ||
| Appearance | White crystalline solid | ||
| Odor | Fishy, ammonia like | ||
| Density | 1.33 g/cm3 (at 20 °C) | ||
| Melting point | 280 °C (536 °F; 553 K) (sublimes) | ||
| 85.3 g/100 mL | |||
| Solubility | Soluble in chloroform, methanol, ethanol, acetone, benzene, xylene, ether | ||
| Solubility in chloroform | 13.4 g/100 g (20 °C) | ||
| Solubility in methanol | 7.25 g/100 g (20 °C) | ||
| Solubility in ethanol | 2.89 g/100 g (20 °C) | ||
| Solubility in acetone | 0.65 g/100 g (20 °C) | ||
| Solubility in benzene | 0.23 g/100 g (20 °C) | ||
| Acidity (pKa) | 4.89 [1] | ||
| Pharmacology | |||
| J01XX05 ( WHO ) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Highly combustible, harmful | ||
| GHS labelling: | |||
  | |||
| Warning | |||
| H228, H317 | |||
| P210, P240, P241, P261, P272, P280, P302+P352, P321, P333+P313, P363, P370+P378, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 250 °C (482 °F; 523 K) | ||
| 410 °C (770 °F; 683 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
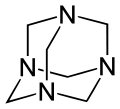
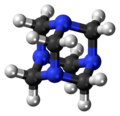
Hexamethylenetetramine (HMTA), also known as 1,3,5,7-tetraazaadamantane, is a heterocyclic organic compound with diverse applications. [2] [3] It has the chemical formula (CH2)6N4 and is a white crystalline compound that is highly soluble in water and polar organic solvents. It is useful in the synthesis of other organic compounds, including plastics, pharmaceuticals, and rubber additives. [2] [3] The compound is also used medically for certain conditions. [4] [5] It sublimes in vacuum at 280 °C. It has a tetrahedral cage-like structure similar to adamantane. [3] The four vertices are occupied by nitrogen atoms, which are linked by methylene groups. Although the molecular shape defines a cage, no void space is available at the interior.