This article is missing information about the history of Polymyxin.(August 2019) |

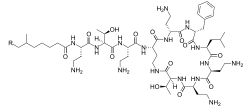
Polymyxins are antibiotics. Polymyxins B and E (also known as colistin) are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
Contents
- Medical use
- Mechanism of action
- Chemistry
- Biosynthesis
- Research
- Compound mixtures in Polymyxin B drug
- See also
- References
They are produced in nature by Gram-positive bacteria such as Paenibacillus polymyxa .

