 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Seromycin |
| Other names | D-cycloserine, 4-amino-3-isoxazolidinone |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~70% to 90% |
| Metabolism | Liver |
| Elimination half-life | 10 hrs (normal kidney function) |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.626 |
| Chemical and physical data | |
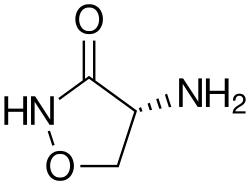
| Formula | C3H6N2O2 |
| Molar mass | 102.093 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 155 to 156 °C (311 to 313 °F) (dec.) |
| |
| |
| (verify) | |
Cycloserine, sold under the brand name Seromycin, is a GABA transaminase inhibitor and an antibiotic, used to treat tuberculosis. [1] [2] Specifically it is used, along with other antituberculosis medications, for active drug resistant tuberculosis. [2] It is given by mouth. [2]
Contents
- Medical uses
- Tuberculosis
- Pharmacology
- Mechanism of action
- Chemistry
- Chemical properties
- Synthesis
- History
- Society and culture
- Economics
- Research
- Psychiatric disorders
- Possible hallucinogenic effects
- References
- Further reading
Common side effects include allergic reactions, seizures, sleepiness, unsteadiness, and numbness. [2] It is not recommended in people who have kidney failure, epilepsy, depression, or are alcoholics. [2] It is unclear if use during pregnancy is safe for the baby. [2] Cycloserine is similar in structure to the amino acid D-alanine and works by interfering with the formation of the bacteria's cell wall. [2]
Cycloserine was discovered in 1954 from a type of Streptomyces . [3] It is on the World Health Organization's List of Essential Medicines. [4]