
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor is an ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The GRIA2-encoded AMPA receptor ligand binding core was the first glutamate receptor ion channel domain to be crystallized.
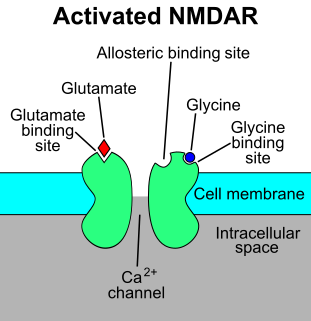
The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a “coincidence detector” and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.

CNQX or cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) is a competitive AMPA/kainate receptor antagonist. Its chemical formula is C9H4N4O4. CNQX is often used in the retina to block the responses of OFF-bipolar cells for electrophysiology recordings.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

Kainate receptors, or kainic acid receptors (KARs), are ionotropic receptors that respond to the neurotransmitter glutamate. They were first identified as a distinct receptor type through their selective activation by the agonist kainate, a drug first isolated from algae. They have been traditionally classified as a non-NMDA-type receptor, along with the AMPA receptor. KARs are less understood than AMPA and NMDA receptors, the other ionotropic glutamate receptors. Postsynaptic kainate receptors are involved in excitatory neurotransmission. Presynaptic kainate receptors have been implicated in inhibitory neurotransmission by modulating release of the inhibitory neurotransmitter GABA through a presynaptic mechanism.

The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.

Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation.

DNQX (6,7-dinitroquinoxaline-2,3-dione) is a competitive antagonist at AMPA and kainate receptors, two ionotropic glutamate receptor (iGluR) subfamilies. It is used in a variety of molecular biology subfields, notably neurophysiology, to assist researchers in determining the properties of various types of ion channels and their potential applications in medicine.

Glutamate receptor 3 is a protein that in humans is encoded by the GRIA3 gene.

Protein Interacting with C Kinase - 1 is a protein that in humans is encoded by the PICK1 gene.

Glutamate receptor 1 is a protein that in humans is encoded by the GRIA1 gene.

Glutamate ionotropic receptor AMPA type subunit 2 is a protein that in humans is encoded by the GRIA2 gene and it is a subunit found in the AMPA receptors.

Glutamate ionotropic receptor kainate type subunit 2, also known as ionotropic glutamate receptor 6 or GluR6, is a protein that in humans is encoded by the GRIK2 gene.

Glutamate receptor, ionotropic, kainate 1, also known as GRIK1, is a protein that in humans is encoded by the GRIK1 gene.

Glutamate receptor 4 is a protein that in humans is encoded by the GRIA4 gene.

Glutamate receptor, ionotropic, delta 2, also known as GluD2, GluRδ2, or δ2, is a protein that in humans is encoded by the GRID2 gene. This protein together with GluD1 belongs to the delta receptor subtype of ionotropic glutamate receptors. They possess 14–24% sequence homology with AMPA, kainate, and NMDA subunits, but, despite their name, do not actually bind glutamate or various other glutamate agonists.

Glutamate receptor, ionotropic kainate 3 is a protein that in humans is encoded by the GRIK3 gene.

Glutamate receptor, ionotropic kainate 5 is a protein that in humans is encoded by the GRIK5 gene.

Willardiine or (S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione is a chemical compound that occurs naturally in the seeds of Mariosousa willardiana and Acacia sensu lato. The seedlings of these plants contain enzymes capable of complex chemical substitutions that result in the formation of free amino acids. Willardiine is frequently studied for its function in higher level plants. Additionally, many derivates of willardiine are researched for their potential in pharmaceutical development. Willardiine was first discovered in 1959 by R. Gmelin, when he isolated several free, non-protein amino acids from Acacia willardiana when he was studying how these families of plants synthesize uracilyalanines. A related compound, Isowillardiine, was concurrently isolated by a different group, and it was discovered that the two compounds had different structural and functional properties. Subsequent research on Willardiine has focused on the functional significance of different substitutions at the nitrogen group and the development of analogs of Willardiine with different pharmacokinetic properties. In general, Willardiine is the one of the first compounds studied in which slight changes to molecular structure result in compounds with significantly different pharmacokinetic properties.



















