Related Research Articles

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

AP5 is a chemical compound used as a biochemical tool to study various cellular processes. It is a selective NMDA receptor antagonist that competitively inhibits the ligand (glutamate) binding site of NMDA receptors. AP5 blocks NMDA receptors in micromolar concentrations.
Ziconotide, sold under the brand name Prialt, also called intrathecal ziconotide (ITZ) because of its administration route, is an atypical analgesic agent for the amelioration of severe and chronic pain. Derived from Conus magus, a cone snail, it is the synthetic form of an ω-conotoxin peptide.

A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.

Tropidolaemus wagleri, more commonly known as Wagler's pit viper, is a species of venomous snake, a pit viper in the subfamily Crotalinae of the family Viperidae. The species is endemic to Southeast Asia. There are no subspecies that are recognized as being valid. It is sometimes referred to as the temple viper because of its abundance around the Temple of the Azure Cloud in Malaysia.
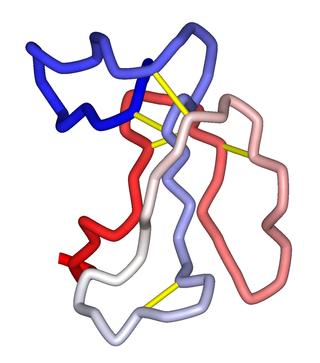
α-Bungarotoxin is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.

Cone snails, or cones, are highly venomous sea snails of the family Conidae.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for humans and animals; the state of anesthesia they induce is referred to as dissociative anesthesia.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channel's initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.

The contryphans are a family of peptides that are active constituents of the potent venom produced by cone snail. The two amino acid cysteine residues in contryphans are linked by a disulfide bond. In addition, contryphans undergo an unusual degree of post-translational modification including epimerization of leucine and tryptophan, tryptophan bromination, amidation of the C-terminus, and proline hydroxylation. In the broader scheme of genetic conotoxin classification, contryphans are members of "Conotoxin Superfamily O2."

Glutamate [NMDA] receptor subunit epsilon-2, also known as N-methyl D-aspartate receptor subtype 2B, is a protein that in humans is encoded by the GRIN2B gene.
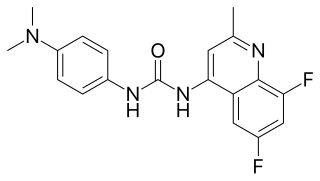
SB-408124 is a drug which is a non-peptide antagonist selective for the orexin receptor subtype OX1, with around 70x selectivity for OX1 over OX2 receptors, and improved oral bioavailability compared to the older OX1 antagonist SB-334867. It is used in scientific research into the function of orexinergic neurons in the body.

Conus ermineus, common name the turtle cone, is a species of sea snail, a marine gastropod mollusc in the family Conidae, the cone snails and their allies.
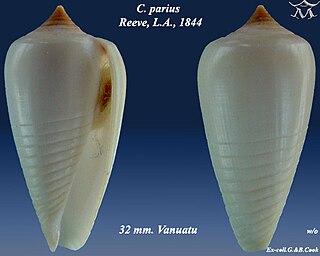
Conus parius, common name the Parian cone, is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.

Conus regius, common name the "crown cone", is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.

Conus victoriae, common name the Queen Victoria cone, is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.
Besonprodil (CI-1041) is a drug which acts as an NMDA antagonist, selective for the NR2B subunit. It is under development as a supplemental medication for Parkinson's disease, and has been shown in animals to be effective in counteracting the dyskinesias associated with long-term treatment with levodopa and related drugs.

Traxoprodil is a drug developed by Pfizer which acts as an NMDA antagonist, selective for the NR2B subunit. It has neuroprotective, analgesic, and anti-Parkinsonian effects in animal studies. Traxoprodil has been researched in humans as a potential treatment to lessen the damage to the brain after stroke, but results from clinical trials showed only modest benefit. The drug was found to cause EKG abnormalities and its clinical development was stopped. More recent animal studies have suggested traxoprodil may exhibit rapid-acting antidepressant effects similar to those of ketamine, although there is some evidence for similar psychoactive side effects and abuse potential at higher doses, which might limit clinical acceptance of traxoprodil for this application.
Leconotide is an ω-conotoxin peptide isolated from the venom of Conus catus which is under investigation as an analgesic drug for the treatment of pain conditions.
CNF-Sr3, also known as conorfamide-Sr3, is a toxin derived from the venom duct of Conus spurius. CNF-Sr3 is an inhibitor of the Shaker channel, a subtype of the voltage-gated potassium channels.
References
- 1 2 Gowd KH, Twede V, Watkins M, Krishnan KS, Teichert RW, Bulaj G, Olivera BM (August 2008). "Conantokin-P, an unusual conantokin with a long disulfide loop". Toxicon. 52 (2): 203–13. Bibcode:2008Txcn...52..203G. doi:10.1016/j.toxicon.2008.04.178. PMC 2630528 . PMID 18586049.
- ↑ Mena EE, Gullak MF, Pagnozzi MJ, Richter KE, Rivier J, Cruz LJ, Olivera BM (October 1990). "Conantokin-G: a novel peptide antagonist to the N-methyl-D-aspartic acid (NMDA) receptor". Neuroscience Letters. 118 (2): 241–4. doi:10.1016/0304-3940(90)90637-O. PMID 2177176. S2CID 32784480.
- 1 2 3 4 5 Wei J, Dong M, Xiao C, Jiang F, Castellino FJ, Prorok M, Dai Q (September 2006). "Conantokins and variants derived from cone snail venom inhibit naloxone-induced withdrawal jumping in morphine-dependent mice". Neuroscience Letters. 405 (1–2): 137–41. doi:10.1016/j.neulet.2006.06.040. PMID 16859831. S2CID 35973753.
- 1 2 3 Malmberg AB, Gilbert H, McCabe RT, Basbaum AI (January 2003). "Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T". Pain. 101 (1–2): 109–16. doi:10.1016/S0304-3959(02)00303-2. PMID 12507705. S2CID 25950992.
- 1 2 3 4 5 Jimenez EC, Donevan S, Walker C, Zhou LM, Nielsen J, Cruz LJ, Armstrong H, White HS, Olivera BM (September 2002). "Conantokin-L, a new NMDA receptor antagonist: determinants for anticonvulsant potency". Epilepsy Research. 51 (1–2): 73–80. doi:10.1016/S0920-1211(02)00101-8. PMID 12350383. S2CID 7960889.
- ↑ Rigby AC, Baleja JD, Li L, Pedersen LG, Furie BC, Furie B (December 1997). "Role of gamma-carboxyglutamic acid in the calcium-induced structural transition of conantokin G, a conotoxin from the marine snail Conus geographus". Biochemistry. 36 (50): 15677–84. doi:10.1021/bi9718550. PMID 9398296.
- ↑ Robinson SD, Norton RS (December 2014). "Conotoxin gene superfamilies". Marine Drugs. 12 (12): 6058–101. doi: 10.3390/md12126058 . PMC 4278219 . PMID 25522317.
- 1 2 Haack JA, Rivier J, Parks TN, Mena EE, Cruz LJ, Olivera BM (April 1990). "Conantokin-T. A gamma-carboxyglutamate containing peptide with N-methyl-d-aspartate antagonist activity". The Journal of Biological Chemistry. 265 (11): 6025–9. doi: 10.1016/S0021-9258(19)39285-3 . PMID 2180939.
- ↑ Olivera BM, McIntosh JM, Clark C, Middlemas D, Gray WR, Cruz LJ (1985). "A sleep-inducing peptide from Conus geographus venom". Toxicon. 23 (2): 277–82. Bibcode:1985Txcn...23..277O. doi:10.1016/0041-0101(85)90150-3. PMID 4024137.
- 1 2 3 Williams AJ, Ling G, McCabe RT, Tortella FC (May 2002). "Intrathecal CGX-1007 is neuroprotective in a rat model of focal cerebral ischemia". NeuroReport. 13 (6): 821–4. doi:10.1097/00001756-200205070-00017. PMID 11997694. S2CID 30052416.
- 1 2 3 4 Alex AB, Baucum AJ, Wilcox KS (September 2006). "Effect of Conantokin G on NMDA receptor-mediated spontaneous EPSCs in cultured cortical neurons". Journal of Neurophysiology. 96 (3): 1084–92. doi:10.1152/jn.01325.2005. PMID 16760339.
- 1 2 3 Teichert RW, Jimenez EC, Twede V, Watkins M, Hollmann M, Bulaj G, Olivera BM (December 2007). "Novel conantokins from Conus parius venom are specific antagonists of N-methyl-D-aspartate receptors". The Journal of Biological Chemistry. 282 (51): 36905–13. doi: 10.1074/jbc.M706611200 . PMID 17962189.
- ↑ Gowd KH, Watkins M, Twede VD, Bulaj GW, Olivera BM (August 2010). "Characterization of conantokin Rl-A: molecular phylogeny as structure/function study". Journal of Peptide Science. 16 (8): 375–82. doi:10.1002/psc.1249. PMC 4136950 . PMID 20572027.
- ↑ Twede VD, Teichert RW, Walker CS, Gruszczyński P, Kaźmierkiewicz R, Bulaj G, Olivera BM (May 2009). "Conantokin-Br from Conus brettinghami and selectivity determinants for the NR2D subunit of the NMDA receptor". Biochemistry. 48 (19): 4063–73. doi:10.1021/bi802259a. PMC 3955384 . PMID 19309162.
- ↑ Ragnarsson L, Mortensen M, Dodd PR, Lewis RJ (May 2002). "Spermine modulation of the glutamate(NMDA) receptor is differentially responsive to conantokins in normal and Alzheimer's disease human cerebral cortex". Journal of Neurochemistry. 81 (4): 765–79. doi: 10.1046/j.1471-4159.2002.00872.x . PMID 12065636.
- ↑ Cnudde SE, Prorok M, Castellino FJ, Geiger JH. (June 2010) “Metal ion determinants of conantokin dimerization as revealed in the X-ray crystallographic structure of the Cd(2+)/Mg (2+)-con-T[K7gamma] complex.” J Biol Inorg Chem.15(5):667-75. Cnudde SE, Prorok M, Castellino FJ, Geiger JH (June 2010). "Metal ion determinants of conantokin dimerization as revealed in the X-ray crystallographic structure of the Cd(2+)/Mg (2+)-con-T[K7gamma] complex". Journal of Biological Inorganic Chemistry. 15 (5): 667–75. doi:10.1007/s00775-010-0633-2. PMC 3693470 . PMID 20195692.
- ↑ Donevan SD, McCabe RT (September 2000). "Conantokin G is an NR2B-selective competitive antagonist of N-methyl-D-aspartate receptors". Molecular Pharmacology. 58 (3): 614–23. doi:10.1124/mol.58.3.614. PMID 10953056.
- ↑ Huang L, Balsara RD, Sheng Z, Castellino FJ (October 2010). "Conantokins inhibit NMDAR-dependent calcium influx in developing rat hippocampal neurons in primary culture with resulting effects on CREB phosphorylation". Molecular and Cellular Neurosciences. 45 (2): 163–72. doi:10.1016/j.mcn.2010.06.007. PMC 2923249 . PMID 20600930.