
Vitamin K is a family of structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation or for controlling binding of calcium in bones and other tissues. The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.

Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia.
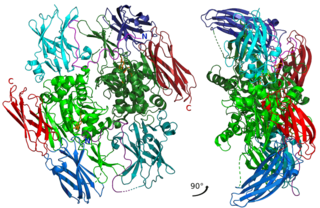
Factor XIII or fibrin stabilizing factor is a zymogen found in blood of humans and some other animals. It is activated by thrombin to factor XIIIa. Factor XIIIa is an enzyme of the blood coagulation system that crosslinks fibrin. Deficiency of XIII worsens clot stability and increases bleeding tendency.
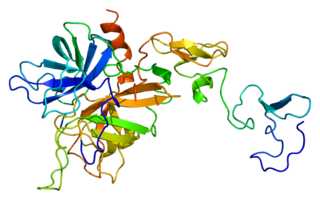
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIX, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the PROC gene, which is found on chromosome 2.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
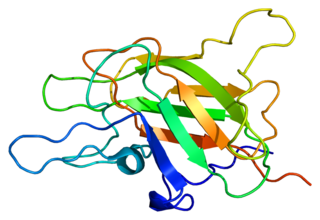
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.

Protein Z is a mammalian protein which is encoded by the PROZ gene.
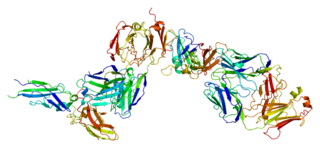
Tissue factor, also called platelet tissue factor, factor III, or CD142, is a protein encoded by the F3 gene, present in subendothelial tissue and leukocytes. Its role in the clotting process is the initiation of thrombin formation from the zymogen prothrombin. Thromboplastin defines the cascade that leads to the activation of factor X—the tissue factor pathway. In doing so, it has replaced the previously named extrinsic pathway in order to eliminate ambiguity.
The prothrombinase complex consists of the serine protease, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (Factor II), an inactive zymogen, to thrombin (Factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that Factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
In coagulation, the procoagulant protein factor X can be activated into factor Xa in two ways: either extrinsically or intrinsically.

Vitamin K epoxide reductase (VKOR) is an enzyme that reduces vitamin K after it has been oxidised in the carboxylation of glutamic acid residues in blood coagulation enzymes. VKOR is a member of a large family of predicted enzymes that are present in vertebrates, Drosophila, plants, bacteria and archaea. In some plant and bacterial homologues, the VKOR domain is fused with domains of the thioredoxin family of oxidoreductases.
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates.

Gamma-glutamyl carboxylase is an enzyme that in humans is encoded by the GGCX gene, located on chromosome 2 at 2p12.

Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.

Matrix Gla protein (MGP) is member of a family of vitamin K2 dependent, Gla-containing proteins. MGP has a high affinity binding to calcium ions, similar to other Gla-containing proteins. The protein acts as an inhibitor of vascular mineralization and plays a role in bone organization.

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.
Peptidyl-glutamate 4-carboxylase (EC 4.1.1.90, vitamin K-dependent carboxylase, gamma-glutamyl carboxylase) is an enzyme with systematic name peptidyl-glutamate 4-carboxylase (2-methyl-3-phytyl-1,4-naphthoquinone-epoxidizing). This enzyme catalyses the following chemical reaction
A Vitamin K-dependent protein (VKDP) is a protein that can bind calcium ions but only after being carboxylated at a certain glutamic residue. This carboxylation, said to activate the protein, is facilitated by some form of vitamin K1 or vitamin K2. The relevant part of a vitamin K-dependent protein is a Gla domain, and such a protein is informally called a Gla protein. Some Gla proteins have "Gla" in their name, for example Matrix Gla protein, but many don't, such as osteocalcin.















