
Vitamin K is a family of structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation or for controlling binding of calcium in bones and other tissues. The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor.
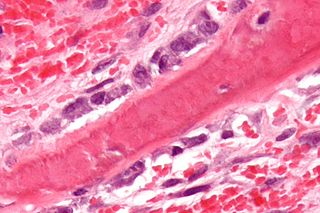
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.

Calcification is the accumulation of calcium salts in a body tissue. It normally occurs in the formation of bone, but calcium can be deposited abnormally in soft tissue, causing it to harden. Calcifications may be classified on whether there is mineral balance or not, and the location of the calcification. Calcification may also refer to the processes of normal mineral deposition in biological systems, such as the formation of stromatolites or mollusc shells.

Osteopontin (OPN), also known as bone /sialoprotein I, early T-lymphocyte activation (ETA-1), secreted phosphoprotein 1 (SPP1), 2ar and Rickettsia resistance (Ric), is a protein that in humans is encoded by the SPP1 gene. The murine ortholog is Spp1. Osteopontin is a SIBLING (glycoprotein) that was first identified in 1986 in osteoblasts.

Osteocalcin, also known as bone gamma-carboxyglutamic acid-containing protein (BGLAP), is a small (49-amino-acid) noncollagenous protein hormone found in bone and dentin, first identified as a calcium-binding protein.

Calciphylaxis, also known as calcific uremic arteriolopathy (CUA) or “Grey Scale”, is a rare syndrome characterized by painful skin lesions. The pathogenesis of calciphylaxis is unclear but believed to involve calcification of the small blood vessels located within the fatty tissue and deeper layers of the skin, blood clots, and eventual death of skin cells due to lack of blood flow. It is seen mostly in people with end-stage kidney disease but can occur in the earlier stages of chronic kidney disease and rarely in people with normally functioning kidneys. Calciphylaxis is a rare but serious disease, believed to affect 1-4% of all dialysis patients. It results in chronic non-healing wounds and indicates poor prognosis, with typical life expectancy of less than one year.

Osteonectin (ON) also known as secreted protein acidic and rich in cysteine (SPARC) or basement-membrane protein 40 (BM-40) is a protein that in humans is encoded by the SPARC gene.
Tartrate-resistant acid phosphatase, also called acid phosphatase 5, tartrate resistant (ACP5), is a glycosylated monomeric metalloprotein enzyme expressed in mammals. It has a molecular weight of approximately 35kDa, a basic isoelectric point (7.6–9.5), and optimal activity in acidic conditions. TRAP is synthesized as latent proenzyme and activated by proteolytic cleavage and reduction. It is differentiated from other mammalian acid phosphatases by its resistance to inhibition by tartrate and by its molecular weight.

Cartilage oligomeric matrix protein (COMP), also known as thrombospondin-5, is an extracellular matrix (ECM) protein primarily present in cartilage. In humans it is encoded by the COMP gene.

Platelet-derived growth factor receptor beta is a protein that in humans is encoded by the PDGFRB gene. Mutations in PDGFRB are mainly associated with the clonal eosinophilia class of malignancies.

Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.

alpha-2-HS-glycoprotein also known as fetuin-A is a protein that in humans is encoded by the AHSG gene. Fetuin-A belongs to the fetuin class of plasma binding proteins and is more abundant in fetal than adult blood.

Cartilage intermediate layer protein 1 is a protein that in humans is encoded by the CILP gene.

S100 calcium-binding protein G (S100G) is a protein that in humans is encoded by the S100G gene.

Chondromodulin-1 is a protein that in humans is encoded by the LECT1 gene.

Transcription factor Sp7, also called Osterix (Osx), is a protein that in humans is encoded by the SP7 gene. It is a member of the Sp family of zinc-finger transcription factors It is highly conserved among bone-forming vertebrate species It plays a major role, along with Runx2 and Dlx5 in driving the differentiation of mesenchymal precursor cells into osteoblasts and eventually osteocytes. Sp7 also plays a regulatory role by inhibiting chondrocyte differentiation maintaining the balance between differentiation of mesenchymal precursor cells into ossified bone or cartilage. Mutations of this gene have been associated with multiple dysfunctional bone phenotypes in vertebrates. During development, a mouse embryo model with Sp7 expression knocked out had no formation of bone tissue. Through the use of GWAS studies, the Sp7 locus in humans has been strongly associated with bone mass density. In addition there is significant genetic evidence for its role in diseases such as Osteogenesis imperfecta (OI).

Keutel syndrome (KS) is a rare autosomal recessive genetic disorder characterized by abnormal diffuse cartilage calcification, hypoplasia of the mid-face, peripheral pulmonary stenosis, hearing loss, short distal phalanges (tips) of the fingers and mild mental retardation. Individuals with KS often present with peripheral pulmonary stenosis, brachytelephalangism, sloping forehead, midface hypoplasia, and receding chin. It is associated with abnormalities in the gene coding for matrix gla protein, MGP. Being an autosomal recessive disorder, it may be inherited from two unaffected, abnormal MGP-carrying parents. Thus, people who inherit two affected MGP alleles will likely inherit KS.
X-linked recessive chondrodysplasia punctata is a type of chondrodysplasia punctata that can involve the skin, hair, and cause short stature with skeletal abnormalities, cataracts, and deafness.

Vitamin K2 or menaquinone (MK) is one of three types of vitamin K, the other two being vitamin K1 (phylloquinone) and K3 (menadione). K2 is both a tissue and bacterial product (derived from vitamin K1 in both cases) and is usually found in animal products or fermented foods.
A disintegrin and metalloproteinase with thrombospondin motifs 7 (ADAMTS7) is an enzyme that in humans is encoded by the ADAMTS7 gene on chromosome 15. It is ubiquitously expressed in many tissues and cell types. This enzyme catalyzes the degradation of cartilage oligomeric matrix protein (COMP). ADAMTS7 has been associated with cancer and arthritis in multiple tissue types. The ADAMTS7 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.





















