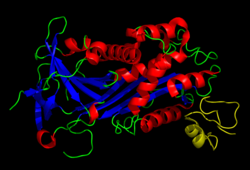
Structure
Vitronectin is a 75 kDa glycoprotein, consisting of 478 amino acid residues. About one-third of the protein's molecular mass is composed of carbohydrates. On occasion, the protein is cleaved after arginine 379, to produce two-chain vitronectin, where the two parts are linked by a disulfide bond. No high-resolution structure has been determined experimentally yet, except for the N-terminal domain.
The protein consists of three domains:
Several structures has been reported for the Somatomedin B domain. The protein was initially crystallized in complex with one of its physiological binding partners: the Plasminogen activator inhibitor-1 (PAI-1) and the structure solved for this complex. [11] Subsequently two groups reported NMR structures of the domain. [12] [13]
The somatomedin B domain is a close-knit disulfide knot, with 4 disulfide bonds within 35 residues. Different disulfide configurations had been reported for this domain [14] [15] [16] but this ambiguity has been resolved by the crystal structure. [16]
Homology models have been built for the central and C-terminal domains. [16]
Function
The somatomedin B domain of vitronectin binds to plasminogen activator inhibitor-1 (PAI-1), and stabilizes it. [11] Thus vitronectin serves to regulate proteolysis initiated by plasminogen activation. In addition, vitronectin is a component of platelets and is, thus, involved in hemostasis. Vitronectin contains an RGD (45-47) sequence, which is a binding site for membrane-bound integrins, e.g., the vitronectin receptor, which serve to anchor cells to the extracellular matrix. The Somatomedin B domain interacts with the urokinase receptor, and this interaction has been implicated in cell migration and signal transduction. High plasma levels of both PAI-1 and the urokinase receptor have been shown to correlate with a negative prognosis for cancer patients. Cell adhesion and migration are directly involved in cancer metastasis, which provides a probable mechanistic explanation for this observation.
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.