Related Research Articles

Collagen is the main structural protein in the extracellular matrix of a body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals. 25% to 35% of a mammalian body's protein content is collagen. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. The collagen helix is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis, while Vitamin E improves its production.

(2S,4R)-4-Hydroxyproline, or L-hydroxyproline (C5H9O3N), is an amino acid, abbreviated as Hyp or O, e.g., in Protein Data Bank.

Ehlers–Danlos syndromes (EDS) are a group of 13 genetic connective-tissue disorders. Symptoms often include loose joints, joint pain, stretchy velvety skin, and abnormal scar formation. These may be noticed at birth or in early childhood. Complications may include aortic dissection, joint dislocations, scoliosis, chronic pain, or early osteoarthritis. The current classification was last updated in 2017, when a number of rarer forms of EDS were added.

Hydroxylysine (Hyl) is an amino acid with the molecular formula C6H14N2O3. It was first discovered in 1921 by Donald Van Slyke as the 5-hydroxylysine form. It arises from a post-translational hydroxy modification of lysine. It is most widely known as a component of collagen.
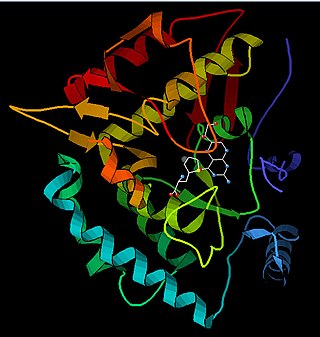
Phenylalanine hydroxylase (PAH) (EC 1.14.16.1) is an enzyme that catalyzes the hydroxylation of the aromatic side-chain of phenylalanine to generate tyrosine. PAH is one of three members of the biopterin-dependent aromatic amino acid hydroxylases, a class of monooxygenase that uses tetrahydrobiopterin (BH4, a pteridine cofactor) and a non-heme iron for catalysis. During the reaction, molecular oxygen is heterolytically cleaved with sequential incorporation of one oxygen atom into BH4 and phenylalanine substrate. In humans, mutations in its encoding gene, PAH, can lead to the metabolic disorder phenylketonuria.
In chemistry, hydroxylation can refer to:

Occipital horn syndrome (OHS), formerly considered a variant of Ehlers–Danlos syndrome, is an X-linked recessive mitochondrial and connective tissue disorder. It is caused by a deficiency in the transport of the essential mineral copper, associated with mutations in the ATP7A gene.

Isocitrate dehydrogenase (IDH) (EC 1.1.1.42) and (EC 1.1.1.41) is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome.

Collagen, type I, alpha 1, also known as alpha-1 type I collagen, is a protein that in humans is encoded by the COL1A1 gene. COL1A1 encodes the major component of type I collagen, the fibrillar collagen found in most connective tissues, including cartilage.
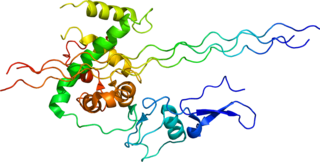
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.
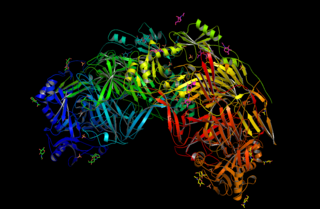
Lysyl oxidase (LOX), also known as protein-lysine 6-oxidase, is an enzyme that, in humans, is encoded by the LOX gene. It catalyzes the conversion of lysine residues into its aldehyde derivative allysine. Allysine form cross-links in extracellular matrix proteins. Inhibition of lysyl oxidase can cause osteolathyrism, but, at the same time, its upregulation by tumor cells may promote metastasis of the existing tumor, causing it to become malignant and cancerous.
Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I forms large, eosinophilic fibers known as collagen fibers, which make up most of the rope-like dense connective tissue in the body.

Tenascin X (TN-X), also known as flexillin or hexabrachion-like protein, is a 450kDa glycoprotein, a member of the tenascin family, that is expressed in connective tissues. In humans it is encoded by the TNXB gene.
Leprecan is a protein associated with osteogenesis imperfecta type VIII.
Type V collagen is a form of fibrillar collagen associated with classical Ehlers-Danlos syndrome. It is found within the dermal/epidermal junction, placental tissues, as well as in association with tissues containing type I collagen.

Collagen alpha-1(V) chain is a protein that in humans is encoded by the COL5A1 gene.

Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2S, 4R)-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome.

Cytochrome P450 family 24 subfamily A member 1 (abbreviated CYP24A1) is a member of the cytochrome P450 superfamily of enzymes encoded by the CYP24A1 gene. It is a mitochondrial monooxygenase which catalyzes reactions including 24-hydroxylation of calcitriol (1,25-dihydroxyvitamin D3). It has also been identified as vitamin D3 24-hydroxylase.(EC 1.14.15.16)

Procollagen-lysine,2-oxoglutarate 5-dioxygenase 3 is an enzyme that in humans is encoded by the PLOD3 gene.

Bruck syndrome is characterized as the combination of arthrogryposis multiplex congenita and osteogenesis imperfecta. Both diseases are uncommon, but concurrence is extremely rare which makes Bruck syndrome very difficult to research. Bruck syndrome is thought to be an atypical variant of osteogenesis imperfecta most resembling type III, if not its own disease. Multiple gene mutations associated with osteogenesis imperfecta are not seen in Bruck syndrome. Many affected individuals are within the same family, and pedigree data supports that the disease is acquired through autosomal recessive inheritance. Bruck syndrome has features of congenital contractures, bone fragility, recurring bone fractures, flexion joint and limb deformities, pterygia, short body height, and progressive kyphoscoliosis. Individuals encounter restricted mobility and pulmonary function. A reduction in bone mineral content and larger hydroxyapatite crystals are also detectable Joint contractures are primarily bilateral and symmetrical, and most prone to ankles. Bruck syndrome has no effect on intelligence, vision, or hearing.
References
- ↑ Hausmann E (Apr 1967). "Cofactor requirements for the enzymatic hydroxylation of lysine in a polypeptide precursor of collagen". Biochimica et Biophysica Acta (BBA) - Protein Structure. 133 (3): 591–3. doi:10.1016/0005-2795(67)90566-1. PMID 6033801.
- ↑ Rhoads RE, Udenfriend S (Aug 1968). "Decarboxylation of alpha-ketoglutarate coupled to collagen proline hydroxylase". Proceedings of the National Academy of Sciences of the United States of America. 60 (4): 1473–8. Bibcode:1968PNAS...60.1473R. doi: 10.1073/pnas.60.4.1473 . PMC 224943 . PMID 5244754.
- ↑ Yeowell, H. N.; Steinmann, B.; Adam, M. P.; Everman, D. B.; Mirzaa, G. M.; Pagon, R. A.; Wallace, S. E.; Bean LJH; Gripp, K. W.; Amemiya, A. (1993). "PLOD1-Related Kyphoscoliotic Ehlers-Danlos Syndrome". University of Washington, Seattle. PMID 20301635.