Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
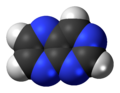
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

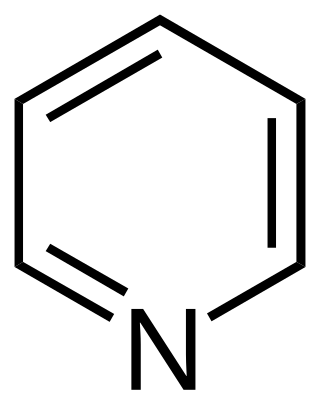
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

Flavins refers generally to the class of organic compounds containing the tricyclic heterocycle isoalloxazine or its isomer alloxazine, and derivatives thereof. The biochemical source of flavin is the yellow B vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide, a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins. Despite the similar names, flavins are chemically and biologically distinct from the flavanoids, and the flavonols.

Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.

Pterin is a heterocyclic compound composed of a pteridine ring system, with a "keto group" and an amino group on positions 4 and 2 respectively. It is structurally related to the parent bicyclic heterocycle called pteridine. Pterins, as a group, are compounds related to pterin with additional substituents. Pterin itself is of no biological significance.
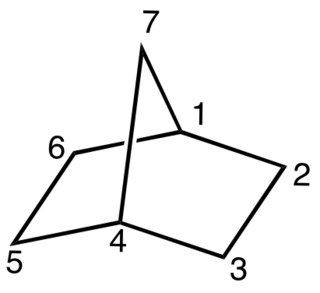
A bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a "deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour".

Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole.
Pyrazole is an organic compound with the formula (CH)3N2H. It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest.
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,3-Triazole is a basic aromatic heterocycle.
In organic chemistry, umpolung or polarity inversion is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

Heterocyclic amines, also sometimes referred to as HCAs, are chemical compounds containing at least one heterocyclic ring, which by definition has atoms of at least two different elements, as well as at least one amine (nitrogen-containing) group. Typically it is a nitrogen atom of an amine group that also makes the ring heterocyclic, though compounds exist in which this is not the case. The biological functions of heterocyclic amines vary, including vitamins and carcinogens. Carcinogenic heterocyclic amines are created by high temperature cooking of meat and smoking of plant matter like tobacco. Some well known heterocyclic amines are niacin, nicotine, and the nucleobases that encode genetic information in DNA.

Biopterins are pterin derivatives which function as endogenous enzyme cofactors in many species of animals and in some bacteria and fungi. The prototypical compound of the class is biopterin, as shown in the infobox. Biopterins act as cofactors for aromatic amino acid hydroxylases (AAAH), which are involved in synthesizing a number of neurotransmitters including dopamine, norepinephrine, epinepherine, and serotonin, along with several trace amines. Nitric oxide synthesis also uses biopterin derivatives as cofactors. In humans, tetrahydrobiopterin (BH4) is the endogenous cofactor for AAAH enzymes.

HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay.
Diazanaphthalenes are a class of aromatic heterocyclic chemical compounds that have the formula C8H6N2. They consist of a naphthalene double ring in which two of the carbon atoms have been replaced with nitrogen atoms. There are ten positional isomers, which differ by the locations of the nitrogen atoms.
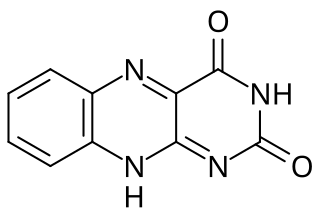
Isoalloxazine is the structural foundation of flavins such as riboflavin and is a heterocyclic compound. It has a tricyclic structure which means it has three interconnected rings of atoms and is a tautomer of alloxazine. The structure is formed by primary-secondary aromatic o-diamines and they are a high-melting crystalline substance. The R-group is used to attach various flavin groups It has a similar structure to pteridines which has two interconnected rings. Isoalloxazine was first obtained in 1934 by Richard Kuhn an Austrian-German biochemist and lab mates.