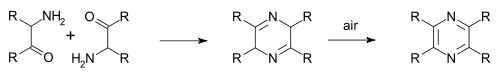
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.
In organic chemistry, the Knoevenagel condensation reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.
The Japp–Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids and aryl diazonium salts. The reaction is named after the chemists Francis Robert Japp and Felix Klingemann.
The Bischler–Möhlau indole synthesis, also often referred to as the Bischler indole synthesis, is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline; it is named after August Bischler and Richard Möhlau .

Friedrich Karl Johannes Thiele was a German chemist and a prominent professor at several universities, including those in Munich and Strasbourg. He developed many laboratory techniques related to isolation of organic compounds. In 1907 he described a device for the accurate determination of melting points, since named Thiele tube after him.

Viktor Meyer was a German chemist and significant contributor to both organic and inorganic chemistry. He is best known for inventing an apparatus for determining vapour densities, the Viktor Meyer apparatus, and for discovering thiophene, a heterocyclic compound. He is sometimes referred to as Victor Meyer, a name used in some of his publications.

Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula C4H4N2. It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other diazine rings, pyrimidine and pyrazine.
In organic chemistry, diazines are a group of organic compounds having the molecular formula C4H4N2. Each contains a benzene ring in which two of the C-H fragments have been replaced by isolobal nitrogen. There are three structural isomers:
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. First described in 1900 by chemists Siegmund Gabriel and James Colman, this rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.
The Koenigs–Knorr reaction in organic chemistry is the substitution reaction of a glycosyl halide with an alcohol to give a glycoside. It is one of the oldest glycosylation reactions. It is named after Wilhelm Koenigs (1851–1906), a student of von Baeyer and fellow student with Hermann Emil Fischer, and Edward Knorr, a student of Koenigs.
In organic chemistry, the Claisen–Schmidt condensation is the reaction between an aldehyde or ketone having an α-hydrogen with an aromatic carbonyl compound lacking an α-hydrogen. It can be considered as a specific variation of the aldol condensation. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. Gustav Schmidt, who independently published on this topic in 1880 and 1881. An example is the synthesis of dibenzylideneacetone ( -1,5-diphenylpenta-1,4-dien-3-one).
The Cook–Heilbron thiazole synthesis highlights the formation of 5-aminothiazoles through the chemical reaction of α-aminonitriles or aminocyanoacetates with dithioacids, carbon disulphide, carbon oxysulfide, or isothiocyanates at room temperature and under mild or aqueous conditions. Variation of substituents at the 2nd and 4th position of the thiazole is introduced by selecting different combinations of starting reagents.
The Dimroth rearrangement is a rearrangement reaction taking place with certain 1,2,3-triazoles where endocyclic and exocyclic nitrogen atoms switch place. This organic reaction was discovered in 1909 by Otto Dimroth.

1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.
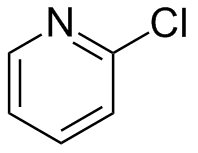
2-Chloropyridine is an aryl chloride with the formula C5H4ClN. It is a colorless liquid that is mainly used to generate fungicides and insecticides in industry. It also serves to generate antihistamines and antiarrythymics for pharmaceutical purposes. It is one of three isomers of chloropyridine.