 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,2-Benzoxazole | |||
| Other names Benzo[d]isoxazole; Indoxazine | |||
| Identifiers | |||
3D model (JSmol) | |||
| 2154 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.440 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C7H5NO | |||
| Molar mass | 119.123 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.18 g/cm3 | ||
| Boiling point | 35 to 38 °C (95 to 100 °F; 308 to 311 K) (at 2.67 hPa) 101-102 °C (at 2 kPa) | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| Flash point | 58 °C (136 °F; 331 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
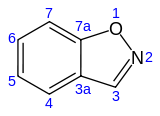
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. [1] [2] The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
Contents
Its aromaticity makes it relatively stable; [3] however, it is only weakly basic.



