 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zonegran, Zonisade |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603008 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% [5] |
| Protein binding | 40% [5] |
| Metabolism | Liver through CYP3A4 [5] |
| Elimination half-life | 63 hours in plasma [5] |
| Excretion | Kidney (62%); Faeces (3%) [5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.118.526 |
| Chemical and physical data | |
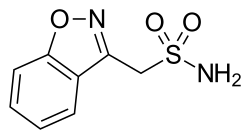
| Formula | C8H8N2O3S |
| Molar mass | 212.22 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 162 °C (324 °F) |
| |
| |
| (verify) | |
Zonisamide, sold under the brand name Zonegran among others, is a medication used to treat the symptoms of epilepsy and Parkinson's disease. [6] [7] Chemically it is a sulfonamide. It serves as an anticonvulsant used primarily as an adjunctive therapy in adults with Parkinson's disease, partial-onset seizures; infantile spasm, mixed seizure types of Lennox–Gastaut syndrome, myoclonic and generalized tonic clonic seizure. [8] Despite this it is also sometimes used as a monotherapy for partial-onset seizures. [7] [9]
Contents
- Medical uses
- Epilepsy
- Parkinson's disease
- Adverse effects
- Interactions
- Mechanism of action
- Pharmacokinetics
- Absorption
- Metabolism
- History
- Research
- Tardive dyskinesia
- Obesity
- Migraine
- Bipolar depression
- References
In 2020, it was the 276th most commonly prescribed medication in the United States, with more than 1 million prescriptions. [10] [11]