Contents
- Chemistry
- Biology
- Biosynthesis and cascade in humans
- PLA2 activation
- PLC activation
- In the body
- Cell membranes
- Brain
- Dietary supplement
- See also
- References
- External links
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name (5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid [1] | |||
| Other names 5,8,11,14-all-cis-Eicosatetraenoic acid all-cis-5,8,11,14-Eicosatetraenoic acid | |||
| Identifiers | |||
3D model (JSmol) | |||
| 1713889 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.007.304 | ||
| EC Number |
| ||
| 58972 | |||
| KEGG | |||
| MeSH | Arachidonic+acid | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C20H32O2 | |||
| Molar mass | 304.474 g·mol−1 | ||
| Density | 0.922 g/cm3 | ||
| Melting point | −49 °C (−56 °F; 224 K) | ||
| Boiling point | 169 to 171 °C (336 to 340 °F; 442 to 444 K) at 0.15 mmHg | ||
| log P | 6.994 | ||
| Acidity (pKa) | 4.752 | ||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H302, H312, H315, H319, H332, H335 | |||
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 113 °C (235 °F; 386 K) | ||
| Related compounds | |||
Related compounds | Eicosatetraenoic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
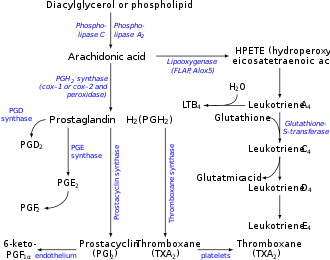
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega−6 fatty acid 20:4(ω−6), or 20:4(5,8,11,14). [2] [3] It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes. [4]
Together with omega−3 fatty acids and other omega−6 fatty acids, arachidonic acid provides energy for body functions, contributes to cell membrane structure, and participates in the synthesis of eicosanoids, which have numerous roles in physiology as signaling molecules. [2] [5]
It was named after the similarly structured Arachidic acid, a constituent of peanut oil whose name in turn derives from the ancient Greek neologism arachis 'peanut'. [6] Peanut oil does not contain any arachidonic acid, itself. [7] Arachidonate is the name of the derived carboxylate anion (conjugate base of the acid), salts, and some esters.