An antipyretic is a substance that reduces fever. Antipyretics cause the hypothalamus to override a prostaglandin-induced increase in temperature. The body then works to lower the temperature, which results in a reduction in fever.

Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be taken orally or intravenously. It typically begins working within an hour.
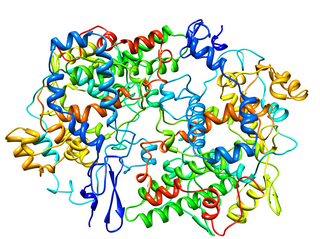
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for biosynthesis of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.

Patent ductus arteriosus (PDA) is a medical condition in which the ductus arteriosus fails to close after birth: this allows a portion of oxygenated blood from the left heart to flow back to the lungs from the aorta, which has a higher blood pressure, to the pulmonary artery, which has a lower blood pressure. Symptoms are uncommon at birth and shortly thereafter, but later in the first year of life there is often the onset of an increased work of breathing and failure to gain weight at a normal rate. With time, an uncorrected PDA usually leads to pulmonary hypertension followed by right-sided heart failure.
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject.
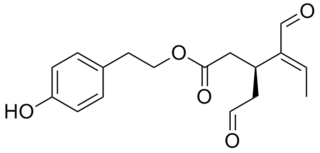
Oleocanthal is a phenylethanoid, or a type of natural phenolic compound found in extra-virgin olive oil. It appears to be responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Oleocanthal is a tyrosol ester and its chemical structure is related to oleuropein, also found in olive oil.
Encaprin was a brand of safety-coated aspirin capsules made by Procter & Gamble in the mid-1980s through its Norwich Eaton Pharmaceuticals division. In 1986, the brand was involved in a cyanide poisoning hoax, and its sales never recovered.

Dexibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). It is the active dextrorotatory enantiomer of ibuprofen. Most ibuprofen formulations contain a racemic mixture of both isomers.

Copper ibuprofenate is a chemical complex consisting of copper(II) and the chelate form of the anti-inflammatory drug ibuprofen. The compound is prepared by the reaction of sodium ibuprofenate with copper(II) sulfate.

Hydrocodone/ibuprofen (INNs), sold under the brand name Vicoprofen, is a fixed-dose combination analgesic medication used in short-term therapy to relieve severe pain. Vicoprofen combines the analgesic and antitussive properties of hydrocodone with the analgesic, anti-inflammatory, and antipyretic properties of ibuprofen. In contrast to hydrocodone/acetaminophen combination analgesics such as Vicodin, this hydrocodone/ibuprofen avoids some of the liver toxicity which may occur from acetaminophen, but still presents significant dangers in hydrocodone overdose, namely respiratory depression. Vicoprofen is supplied in a fixed dose combination tablet which contains hydrocodone bitartrate, USP 7.5 mg with ibuprofen, USP 200 mg. Additional strengths of generic Vicoprofen are now available, in combinations of 5 mg/200 mg and 10 mg/200 mg respectively.
Ibuprofen/paracetamol, sold under the brand name Combogesic among others, is a fixed-dose combination of two medications, ibuprofen, a non-steroidal anti-inflammatory drug (NSAID); and paracetamol (acetaminophen), an analgesic and antipyretic. It is available as a generic medication.

Tripalmitin is a triglyceride derived from the fatty acid palmitic acid.
Nurofen is a brand of range of pain-relief medication containing ibuprofen made by the English-Dutch company Reckitt Benckiser. Introduced in 1983, the Nurofen brand was acquired following Reckitt Benckiser's acquisition of Boots healthcare international in 2005 for £1.93 billion, which included Nurofen, Strepsils, and Clearasil. The brand is primarily marketed and sold in the United Kingdom, other parts of Europe, South Africa, Australia and New Zealand. In 2016, it was the biggest selling branded over-the-counter medication sold in Great Britain, with sales of £116.8 million.
Stewart Sanders Adams was an English pharmacist, and bioengineer who was part of a team from Boots which developed the painkiller ibuprofen in 1961. Ibuprofen is now on the World Health Organization's Model List of Essential Medicines and is one of the world's best-selling drugs.
Oxycodone/ibuprofen is an oral combination drug formulation of the opioid analgesic oxycodone and the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen that is used in the treatment of chronic and acute pain. This particular drug is supplied in a fixed dose combination tablet which contains Oxycodone Hydrochloride, USP 5 mg with Ibuprofen, USP 400 mg.
Ibuprofen/famotidine, sold under the brand name Duexis, is a fixed-dose combination medication used for the treatment of rheumatoid arthritis and osteoarthritis. It contains ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID) and famotidine, a histamine H2-receptor antagonist.
Gadolinium(III) hydroxide is a chemical compound with the formula Gd(OH)3. Its nanoparticles has a potential use for layering various drugs, such as diclofenac, ibuprofen, and naproxen.










