
An analgesic drug, also called simply an analgesic, antalgic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.

Ketoprofen is one of the propionic acid class of nonsteroidal anti-inflammatory drugs (NSAID) with analgesic and antipyretic effects. It acts by inhibiting the body's production of prostaglandin.

Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.

Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work on opioid receptors in the brain and other organs to produce a variety of morphine-like effects, including pain relief.
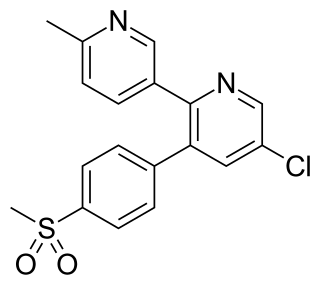
Etoricoxib, sold under the brand name Arcoxia, is a selective COX-2 inhibitor developed and commercialized by Merck. It is approved in 63 countries worldwide as of 2007, except the United States where the Food and Drug Administration sent a Non Approvable Letter to Merck and required them to provide additional data.

Orphenadrine is an anticholinergic drug of the ethanolamine antihistamine class; it is closely related to diphenhydramine. It is a muscle relaxant that is used to treat muscle pain and to help with motor control in Parkinson's disease, but has largely been superseded by newer drugs. It is considered a dirty drug due to its multiple mechanisms of action in different pathways. It was discovered and developed in the 1940s.

Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.

Levonantradol (CP 50,556-1) is a synthetic cannabinoid analog of dronabinol (Marinol) developed by Pfizer in the 1980s. It is around 30 times more potent than THC, and exhibits antiemetic and analgesic effects via activation of CB1 and CB2 cannabinoid receptors. Levonantradol is not currently used in medicine as dronabinol or nabilone are felt to be more useful for most conditions, however it is widely used in research into the potential therapeutic applications of cannabinoids.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.
Tolmetin is a nonsteroidal anti-inflammatory drug (NSAID) of the heterocyclic acetic acid derivative class.
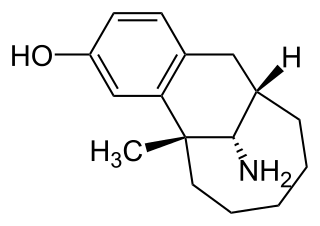
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
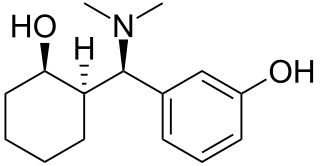
Ciramadol (WY-15,705) is an opioid analgesic that was developed in the late 1970s and is related to phencyclidine, tramadol, tapentadol and venlafaxine. It is a mixed agonist-antagonist for the μ-opioid receptor with relatively low abuse potential and a ceiling on respiratory depression which makes it a relatively safe drug. It has a slightly higher potency and effectiveness as an analgesic than codeine, but is weaker than morphine. Other side effects include sedation and nausea but these are generally less severe than with other similar drugs.
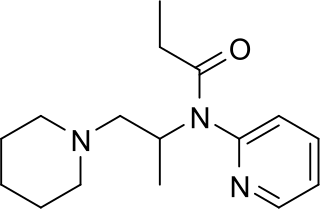
Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Bicifadine (DOV-220,075) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) discovered at American Cyanamid as an analgesic drug candidate, and licensed to DOV Pharmaceutical in 1998 after American Cyanamid was acquired by Wyeth.

Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, with central stimulant and sympathomimetic properties that is primarily used to treat moderate to severe pain.

Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic that was originally used as an analgesic for acute and chronic pain but in 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers. In March 2018, marketing authorisations for flupirtine were withdrawn following a European Medicines Agency recommendation based on the finding that the restrictions introduced in 2013 had not been sufficiently followed in clinical practice, and cases of serious liver injury still occurred including liver failure.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others.

Doxpicomine is a mild opioid analgesic drug. The drug acts as a mu-opioid receptor agonist. It is of fairly low potency, with a 400 mg dose of doxpicomine approximately equivalent in pain-killing effect to 8 mg morphine or 100 mg pethidine. It has been used as a lead compound to derive further analogues, although all compounds in this family are comparatively weak mu agonists.
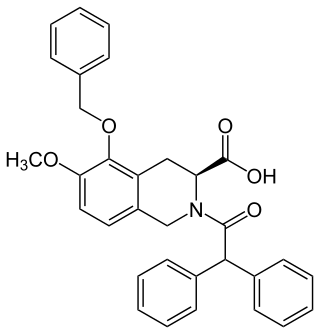
EMA401 is a drug under development for the treatment of peripheral neuropathic pain. Trials were discontinued in 2015, with new trials scheduled to begin March, 2018. It was initially established as a potential drug option for patients suffering pain caused by postherpetic neuralgia. It may also be useful for treating various types of chronic neuropathic pain EMA401 has shown efficacy in preclinical models of shingles, diabetes, osteoarthritis, HIV and chemotherapy. EMA401 is a competitive antagonist of angiotensin II type 2 receptor (AT2R) being developed by the Australian biotechnology company Spinifex Pharmaceuticals. EMA401 target angiotensin II type 2 receptors, which may have importance for painful sensitisation.

Pain management in children is the assessment and treatment of pain in infants and children.




















