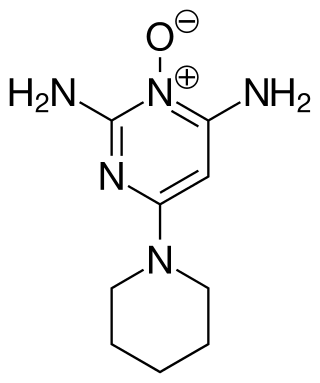
Minoxidil is a medication used for the treatment of high blood pressure and pattern hair loss. It is an antihypertensive vasodilator. It is available as a generic medication by prescription in oral tablet form and over the counter as a topical liquid or foam.

Ketoconazole, sold under the brand name Nizoral among others, is an antiandrogen, antifungal, and antiglucocorticoid medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrheic dermatitis. Taken by mouth it is a less preferred option and only recommended for severe infections when other agents cannot be used. Other uses include treatment of excessive male-patterned hair growth in women and Cushing's syndrome.

Finasteride, sold under the brand names Proscar and Propecia among others, is a medication used to treat hair loss and benign prostatic hyperplasia (BPH) in men. It can also be used to treat excessive hair growth in women. It is taken orally.
The management of hair loss, includes prevention and treatment of alopecia, baldness, and hair thinning, and regrowth of hair.

Pattern hair loss (also known as androgenetic alopecia (AGA)) is a hair loss condition that primarily affects the top and front of the scalp. In male-pattern hair loss (MPHL), the hair loss typically presents itself as either a receding front hairline, loss of hair on the crown (vertex) of the scalp, or a combination of both. Female-pattern hair loss (FPHL) typically presents as a diffuse thinning of the hair across the entire scalp.
A prostaglandin antagonist is a hormone antagonist acting upon one or more prostaglandins, a subclass of eicosanoid compounds which function as signaling molecules in numerous types of animal tissues.

Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2). It is a major prostaglandin produced by mast cells – recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large amounts of PGD2 are found only in the brain and in mast cells. It is critical to development of allergic diseases such as asthma. Research carried out in 1989 found PGD2 is the primary mediator of vasodilation (the "niacin flush") after ingestion of niacin (nicotinic acid).

The Prostaglandin D2 receptor 1 (DP1), a G protein-coupled receptor encoded by the PTGDR1 gene (also termed PTGDR), is primarily a receptor for prostaglandin D2 (PGD2). The receptor is a member of the Prostaglandin receptors belonging to the Subfamily A14 of rhodopsin-like receptors. Activation of DP1 by PGD2 or other cognate receptor ligands is associated with a variety of physiological and pathological responses in animal models.

Prostaglandin-H2 D-isomerase (PTGDS) is an enzyme that in humans is encoded by the PTGDS gene.
Cyclopentenone prostaglandins are a subset of prostaglandins (PGs) or prostanoids that has 15-deoxy-Δ12,14-prostaglandin J2 (15-d-Δ12,14-PGJ2), Δ12-PGJ2, and PGJ2 as its most prominent members but also including PGA2, PGA1, and, while not classified as such, other PGs. 15-d-Δ12,14-PGJ2, Δ12-PGJ2, and PGJ2 share a common mono-unsaturated cyclopentenone structure as well as a set of similar biological activities including the ability to suppress inflammation responses and the growth as well as survival of cells, particularly those of cancerous or neurological origin. Consequently, these three cyclopentenone-PGs and the two epoxyisoprostanes are suggested to be models for the development of novel anti-inflammatory and anti-cancer drugs. The cyclopenentone prostaglandins are structurally and functionally related to a subset of isoprostanes viz., two cyclopentenone isoprostanes, 5,6-epoxyisoprostane E2 and 5,6-epoxisoprostane A2.

In enzymology, a prostaglandin-D synthase is an enzyme that catalyzes the chemical reaction

Prostaglandin D2 receptor 2 (DP2 or CRTH2) is a human protein encoded by the PTGDR2 gene and GPR44. DP2 has also been designated as CD294 (cluster of differentiation 294). It is a member of the class of prostaglandin receptors which bind with and respond to various prostaglandins. DP2 along with Prostaglandin DP1 receptor are receptors for prostaglandin D2 (PGD2). Activation of DP2 by PGD2 or other cognate receptor ligands has been associated with certain physiological and pathological responses, particularly those associated with allergy and inflammation, in animal models and certain human diseases.

Prostaglandin F receptor (FP) is a receptor belonging to the prostaglandin (PG) group of receptors. FP binds to and mediates the biological actions of Prostaglandin F2α (PGF2α). It is encoded in humans by the PTGFR gene.

The Prostacyclin receptor, also termed the prostaglandin I2 receptor or just IP, is a receptor belonging to the prostaglandin (PG) group of receptors. IP binds to and mediates the biological actions of prostacyclin (also termed Prostaglandin I2, PGI2, or when used as a drug, epoprostenol). IP is encoded in humans by the PTGIR gene. While possessing many functions as defined in animal model studies, the major clinical relevancy of IP is as a powerful vasodilator: stimulators of IP are used to treat severe and even life-threatening diseases involving pathological vasoconstriction.

Laropiprant (INN) was a drug used in combination with niacin to reduce blood cholesterol that is no longer sold, due to increases in side-effects with no cardiovascular benefit. Laropiprant itself has no cholesterol lowering effect, but it reduces facial flushes induced by niacin.

Ramatroban (INN) is a thromboxane receptor antagonist.
Non scarring hair loss, also known as noncicatricial alopecia is the loss of hair without any scarring being present. There is typically little inflammation and irritation, but hair loss is significant. This is in contrast to scarring hair loss during which hair follicles are replaced with scar tissue as a result of inflammation. Hair loss may be spread throughout the scalp (diffuse) or at certain spots (focal). The loss may be sudden or gradual with accompanying stress.

Topilutamide, known more commonly as fluridil and sold under the brand name Eucapil, is an antiandrogen medication which is used in the treatment of pattern hair loss in men and women. It is used as a topical medication and is applied to the scalp. Topilutamide belongs to a class of molecules known as perfluoroacylamido-arylpropanamides.
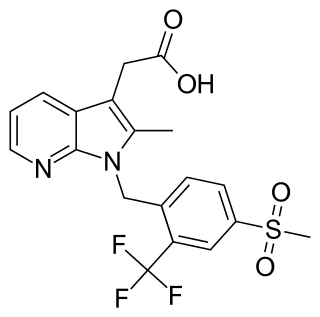
Fevipiprant (INN; code name QAW039) is a drug being developed by Novartis which acts as a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2 or CRTh2).

Pyrilutamide is a nonsteroidal antiandrogen (NSAA) – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR) – which is under development by Suzhou Kintor Pharmaceuticals, inc., a subsidiary of Kintor Pharmaceutical Limited, for the potential treatment of androgenic alopecia and acne in China and the United States. As of October 2022, it is in phase 3 clinical trials for androgenic alopecia and phase 2 trials for acne.















