Related Research Articles
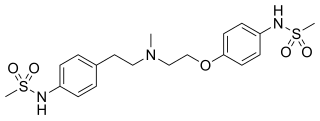
Dofetilide is a class III antiarrhythmic agent. It is marketed under the trade name Tikosyn by Pfizer, and is available in the United States in capsules containing 125, 250, and 500 μg of dofetilide. It is not available in Europe or Australia.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.

Cetirizine is a second-generation antihistamine used to treat allergic rhinitis, dermatitis, and urticaria (hives). It is taken by mouth. Effects generally begin within thirty minutes and last for about a day. The degree of benefit is similar to other antihistamines such as diphenhydramine, which is a first-generation antihistamine.

Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
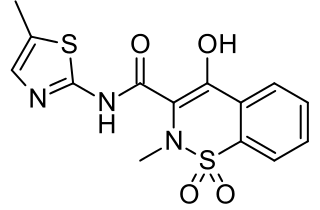
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is used by mouth or by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

Armodafinil, sold under the brand name Nuvigil, is a wakefulness-promoting medication which is used to treat excessive daytime sleepiness associated with obstructive sleep apnea, narcolepsy, and shift work disorder. It is also used off-label for certain other indications. The drug is taken by mouth.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.

Trospium chloride is a muscarinic antagonist used to treat overactive bladder. It has side effects typical of this class of drugs, namely dry mouth, stomach upset, and constipation; these side effects cause problems with people taking their medicine as directed. However it doesn't cause central nervous system side effects like some other muscarinic antagonists. It is in pregnancy category C and is excreted in breast milk.
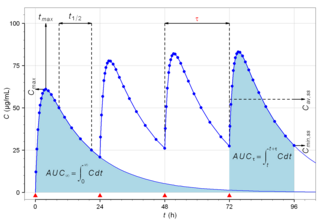
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to describing how the body affects a specific substance after administration. The substances of interest include any chemical xenobiotic such as pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is based on mathematical modeling that places great emphasis on the relationship between drug plasma concentration and the time elapsed since the drug's administration. Pharmacokinetics is the study of how an organism affects the drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.

Delorazepam, also known by the synonyms chlordesmethyldiazepam and nordiclazepam, is a drug which is a benzodiazepine and a derivative of desmethyldiazepam. It is marketed in Italy, where it is available under the trade name EN and Dadumir. Delorazepam (chlordesmethyldiazepam) is also an active metabolite of the benzodiazepine drugs diclazepam and cloxazolam. Adverse effects may include hangover type effects, drowsiness, behavioural impairments and short-term memory impairments. Similar to other benzodiazepines delorazepam has anxiolytic, skeletal muscle relaxant, hypnotic and anticonvulsant properties.
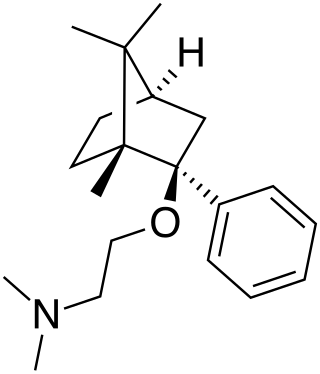
Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.
Cmin is a term used in pharmacokinetics for the minimum blood plasma concentration reached by a drug during a dosing interval, which is the time interval between administration of two doses. This definition is slightly different from Ctrough, the concentration immediately prior to administration of the next dose. Cmin is the opposite of Cmax, the maximum concentration that the drug reaches. Cmin must be above certain thresholds, such as the minimum inhibitory concentration (MIC), to achieve a therapeutic effect.
In the field of pharmacokinetics, the area under the curve (AUC) is the definite integral of the concentration of a drug in blood plasma as a function of time. In practice, the drug concentration is measured at certain discrete points in time and the trapezoidal rule is used to estimate AUC. In pharmacology, the area under the plot of plasma concentration of a drug versus time after dosage gives insight into the extent of exposure to a drug and its clearance rate from the body.
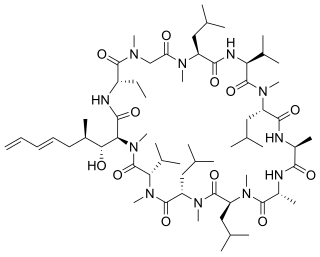
Voclosporin, sold under the brand name Lupkynis, is a calcineurin inhibitor used as an immunosuppressant medication for the treatment of lupus nephritis. It is an analog of ciclosporin that has enhanced action against calcineurin and greater metabolic stability.

Gabapentinoids, also known as α2δ ligands, are a class of drugs that are derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) which block α2δ subunit-containing voltage-dependent calcium channels (VDCCs). This site has been referred to as the gabapentin receptor, as it is the target of the drugs gabapentin and pregabalin.

Lomerizine (INN) is a diphenylpiperazine class L-type and T-type calcium channel blocker. This drug is currently used clinically for the treatment of migraines, while also being used experimentally for the treatment of glaucoma and optic nerve injury.

Eluxadoline, sold under the brand names Viberzi and Truberzi, is a medication taken by mouth for the treatment of diarrhea and abdominal pain in individuals with diarrhea-predominant irritable bowel syndrome (IBS-D). It was approved for use in the United States in 2015. The drug originated from Janssen Pharmaceutica and was developed by Actavis.

The pharmacokinetics of progesterone, concerns the pharmacodynamics, pharmacokinetics, and various routes of administration of progesterone.

Grapiprant, sold under the brand name Galliprant, is a small molecule drug that belongs in the piprant class. This analgesic and anti-inflammatory drug is primarily used as a pain relief for mild to moderate inflammation related to osteoarthritis in dogs. Grapiprant has been approved by the FDA's Center for Veterinary Medicine and was categorized as a non-cyclooxygenase inhibiting non-steroidal anti-inflammatory drug (NSAID) in March 2016.
Dasiglucagon, sold under the brand name Zegalogue, is a medication used to treat severe hypoglycemia in people with diabetes.
References
- ↑ Tracy TS (2004). "Pharmacokinetics". In Stitzel RE, Craig CF (eds.). Modern pharmacology with clinical applications. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 49. ISBN 0-7817-3762-1.
- ↑ Amy Newlands. "Statistics and Pharmacokinetics in Clinical Pharmacology Studies" (PDF). Archived from the original (PDF) on 2013-11-13.
- ↑ Urso R, Blardi P, Giorgi G (March 2002). "A short introduction to pharmacokinetics". Eur Rev Med Pharmacol Sci. 6 (2–3): 33–44. PMID 12708608.
- ↑ Midha KK, Rawson MJ, Hubbard JW (October 2005). "The bioequivalence of highly variable drugs and drug products". Int J Clin Pharmacol Ther. 43 (10): 485–98. doi:10.5414/cpp43485. PMID 16240706.
- ↑ "Bioavailability and Bioequivalence Studies for Orally Administered Drug Products — General Considerations". FDA. Retrieved 18 February 2021.