 | |
| Names | |
|---|---|
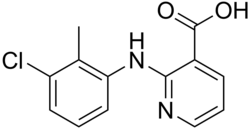
| Preferred IUPAC name 2-(3-Chloro-2-methylanilino)pyridine-3-carboxylic acid | |
| Other names Clonixic acid; CBA 93626 [1] | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.037.921 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C13H11ClN2O2 | |
| Molar mass | 262.69 g·mol−1 |
| Pharmacology | |
| per os | |
| Pharmacokinetics: | |
| Glucuronidation via UGT2B7 | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Clonixin is a nonsteroidal anti-inflammatory drug (NSAID). It also has analgesic, antipyretic, and platelet-inhibitory actions. It is used primarily in the treatment of chronic arthritic conditions and certain soft tissue disorders associated with pain and inflammation.

