Contents
 | |
| Names | |
|---|---|
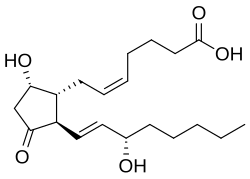
| IUPAC name 9α,15S-Dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.741 |
| KEGG | |
| MeSH | Prostaglandin+D2 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C20H32O5 | |
| Molar mass | 352.471 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2). [1] [2] It is a major prostaglandin produced by mast cells – recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large amounts of PGD2 are found only in the brain and in mast cells. It is critical to development of allergic diseases such as asthma. Research carried out in 1989 [3] found PGD2 is the primary mediator of vasodilation (the "niacin flush") after ingestion of niacin (nicotinic acid).
A 2012 research paper indicates a causal link between elevated levels of localized PGD2 and hair growth inhibition. [4] Applied topically, the researchers found PGD2 prevents hair growth, and mice that were genetically inclined to produce higher levels of PGD2 had inhibited hair growth. The researchers also found PGD2 levels were much higher in balding scalp tissue than nonbalding scalp tissue, through increased levels of prostaglandin D2 synthase. The paper suggested that inhibition of hair growth involved binding of PGD2 to a DP2 receptor, and that DP2 therefore would be a therapeutic target for androgenic alopecia in both men and women with hair loss and thinning. [5] Because PGD2's relation to asthma has been known for several years, several drugs that seek to reduce the effect of PGD2 through blocking the DP2 are already in clinical trials. [5]