This article needs additional citations for verification .(October 2021) |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Remodulin, Orenitram, Tyvaso, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a622038 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous, intravenous, inhalation, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Substantially metabolized by the liver |
| Elimination half-life | 4 hours |
| Excretion | Urine (79% of administered dose is excreted as 4% unchanged drug and 64% as identified metabolites); feces (13%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.236.149 |
| Chemical and physical data | |
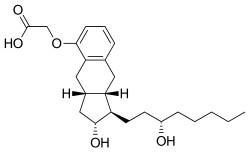
| Formula | C23H34O5 |
| Molar mass | 390.520 g·mol−1 |
| |
| | |
Treprostinil, sold under the brand names Remodulin for infusion, Orenitram for oral, and Tyvaso for inhalation among others, is a vasodilator that is used for the treatment of pulmonary arterial hypertension. [7]
Contents
Treprostinil was approved for use in the United States in May 2002. [8]