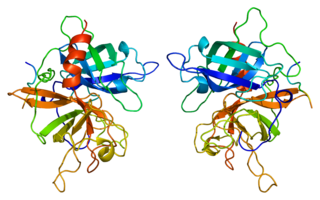A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to prevent bleeding, but can be harmful in thrombosis, when clots obstruct blood flow through healthy blood vessels.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.
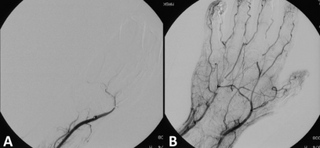
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.
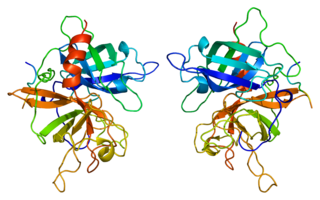
Tissue plasminogen activator is a protein involved in the breakdown of blood clots. It is a serine protease found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Human tPA has a molecular weight of ~70 kDa in the single-chain form.
Streptokinase (SK) is a thrombolytic medication and enzyme. As a medication it is used to break down clots in some cases of myocardial infarction, pulmonary embolism, and arterial thromboembolism. The type of heart attack it is used in is an ST elevation myocardial infarction (STEMI). It is given by injection into a vein.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.

Alteplase (t-PA) is a thrombolytic medication, used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.

Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.

A cerebral infarction is the pathologic process that results in an area of necrotic tissue in the brain. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia), most commonly due to thromboembolism, and manifests clinically as ischemic stroke. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.
Ultrasound-enhanced systemic thrombolysis (UEST) is a medical technology that uses ultrasound to enhance the effects of thrombolytic drugs. To treat the blood clots causing strokes, transcranial Doppler ultrasonography is used. It is thought that transcranial doppler ultrasonography aimed at residual obstructive intracranial blood flow may help expose thrombi to tissue plasminogen activator or other thrombolytic drugs.
Tenecteplase is an enzyme used as a thrombolytic drug.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.
Staphylokinase is a protein produced by Staphylococcus aureus. It contains 136 amino acid residues and has a molecular mass of 15kDa. Synthesis of staphylokinase occurs in late exponential phase. It is similar to streptokinase.
The Thrombolysis In Myocardial Infarction, or TIMI Study Group, is an Academic Research Organization (ARO) affiliated with Brigham and Women's Hospital and Harvard Medical School dedicated to advancing the knowledge and care of patients suffering from cardiovascular disease. The TIMI Study Group provides robust expertise in the key aspects of a clinical trial, including academic leadership, global trial management, biostatistics, clinical event adjudication, safety desk, medical hotline, and core laboratories. The group has its headquarters in Boston, Massachusetts.
The International Studies of Infarct Survival (ISIS) were four randomized controlled trials of several drugs for treating suspected acute myocardial infarction. More than 134,000 patients from over 20 countries took part in four large simple trials between 1981 and 1993, coordinated from Oxford, England.
The fibrinolysis system is responsible for removing blood clots. Hyperfibrinolysis describes a situation with markedly enhanced fibrinolytic activity, resulting in increased, sometimes catastrophic bleeding. Hyperfibrinolysis can be caused by acquired or congenital reasons. Among the congenital conditions for hyperfibrinolysis, deficiency of alpha-2-antiplasmin or plasminogen activator inhibitor type 1 (PAI-1) are very rare. The affected individuals show a hemophilia-like bleeding phenotype. Acquired hyperfibrinolysis is found in liver disease, in patients with severe trauma, during major surgical procedures, and other conditions. A special situation with temporarily enhanced fibrinolysis is thrombolytic therapy with drugs which activate plasminogen, e.g. for use in acute ischemic events or in patients with stroke. In patients with severe trauma, hyperfibrinolysis is associated with poor outcome. Moreover, hyperfibrinolysis may be associated with blood brain barrier impairment, a plasmin-dependent effect due to an increased generation of bradykinin.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), followed by a coronary angioplasty. The angioplasty uses the insertion of a balloon to open up the artery, with the possible additional use of one or more stents. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.

Management of acute coronary syndrome is targeted against the effects of reduced blood flow to the afflicted area of the heart muscle, usually because of a blood clot in one of the coronary arteries, the vessels that supply oxygenated blood to the myocardium. This is achieved with urgent hospitalization and medical therapy, including drugs that relieve chest pain and reduce the size of the infarct, and drugs that inhibit clot formation; for a subset of patients invasive measures are also employed. Basic principles of management are the same for all types of acute coronary syndrome. However, some important aspects of treatment depend on the presence or absence of elevation of the ST segment on the electrocardiogram, which classifies cases upon presentation to either ST segment elevation myocardial infarction (STEMI) or non-ST elevation acute coronary syndrome (NST-ACS); the latter includes unstable angina and non-ST elevation myocardial infarction (NSTEMI). Treatment is generally more aggressive for STEMI patients, and reperfusion therapy is more often reserved for them. Long-term therapy is necessary for prevention of recurrent events and complications.
A migrainous infarction is a rare type of ischaemic stroke which occurs in correspondence with migraine aura symptoms. Symptoms include headaches, visual disturbances, strange sensations and dysphasia, all of which gradually worsen causing neurological changes which ultimately increase the risk of an ischaemic stroke. Typically, women under the age of 45 who experience migraine with aura (MA) are at the greatest risk for developing migrainous infarction, especially when combined with smoking and use of oral contraceptives.