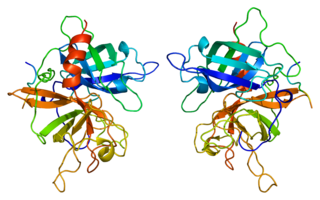Vampire bats, members of the subfamily Desmodontinae, are leaf-nosed bats currently found in Central and South America. Their food source is the blood of other animals, a dietary trait called hematophagy. Three extant bat species feed solely on blood: the common vampire bat, the hairy-legged vampire bat, and the white-winged vampire bat. Two extinct species of the genus Desmodus have been found in North America.

Ischemia or ischaemia is a restriction in blood supply to any tissue, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism. Ischemia is generally caused by problems with blood vessels, with resultant damage to or dysfunction of tissue i.e. hypoxia and microvascular dysfunction. It also implies local hypoxia in a part of a body resulting from constriction. Ischemia causes not only insufficiency of oxygen, but also reduced availability of nutrients and inadequate removal of metabolic wastes. Ischemia can be partial or total blockage. The inadequate delivery of oxygenated blood to the organs must be resolved either by treating the cause of the inadequate delivery or reducing the oxygen demand of the system that needs it. For example, patients with myocardial ischemia have a decreased blood flow to the heart and are prescribed with medications that reduce chronotrophy and ionotrophy to meet the new level of blood delivery supplied by the stenosed vasculature so that it is adequate.
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.
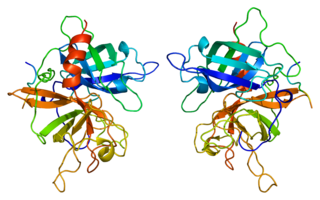
Tissue-type plasminogen activator, short name tPA, is a protein involved in the breakdown of blood clots. It is encoded in the human by the PLAT gene. It is a serine protease found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Human tPA has a molecular weight of ~70 kDa in the single-chain form.
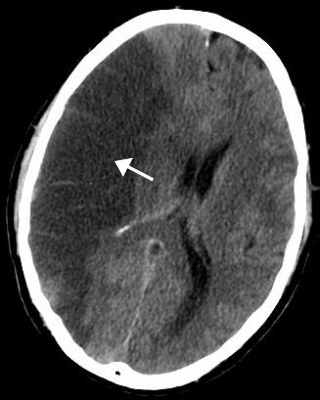
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly.
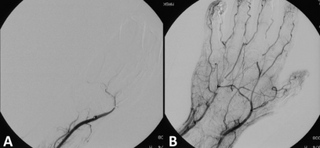
Mechanical thrombectomy, or simply thrombectomy, is the removal of a blood clot (thrombus) from a blood vessel, often and especially endovascularly as an interventional radiology procedure called endovascular thrombectomy (EVT). It thus contrasts with thrombolysis by thrombolytic medications, as either alternative or complement thereto. It is commonly performed in the cerebral arteries as treatment to reverse the ischemia in some ischemic strokes. Open vascular surgery versions of thrombectomy also exist. The effectiveness of thrombectomy for strokes was confirmed in several randomised clinical trials conducted at various medical centers throughout the United States, as reported in a seminal multistudy report in 2015.
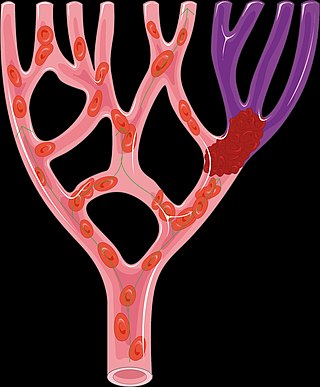
Alteplase, sold under the brand name Activase among others, is a biosynthetic form of human tissue-type plasminogen activator (t-PA). It is a thrombolytic medication used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.
Dias or DIAS may refer to:

Cerebral infarction is the pathologic process that results in an area of necrotic tissue in the brain. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia), most commonly due to thromboembolism, and manifests clinically as ischemic stroke. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.
Ancrod is a defibrinogenating agent derived from the venom of the Malayan pit viper. Defibrinogenating blood produces an anticoagulant effect. Ancrod is not approved or marketed in any country. It is a thrombin-like serine protease.
Tenecteplase, sold under the trade names TNKase, Metalyse and Elaxim, is an enzyme used as a thrombolytic drug.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.
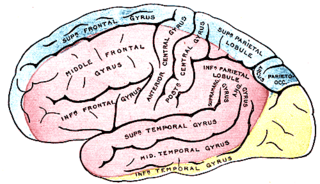
Anterior cerebral artery syndrome is a condition whereby the blood supply from the anterior cerebral artery (ACA) is restricted, leading to a reduction of the function of the portions of the brain supplied by that vessel: the medial aspects of the frontal and parietal lobes, basal ganglia, anterior fornix and anterior corpus callosum.
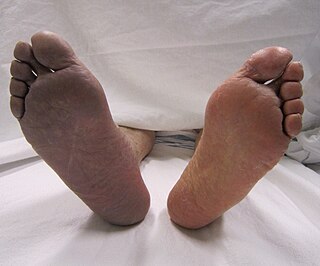
Acute limb ischaemia (ALI) occurs when there is a sudden lack of blood flow to a limb, within 14 days of symptoms onset. It is different from another condition which is more chronic called critical limb ischemia (CLD). CLD is the end stage of peripheral vascular disease where there is still some collateral circulation (alternate circulation pathways} that bring some blood to the distal parts of the limbs. While limbs in both acute and chronic limb ischemia may be pulseless, a chronically ischemic limb is typically warm and pink due to a well-developed collateral artery network and does not need emergency intervention to avoid limb loss.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon and/or stents to open up the artery. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.

Hematophagy is the practice by certain animals of feeding on blood. Since blood is a fluid tissue rich in nutritious proteins and lipids that can be taken without great effort, hematophagy is a preferred form of feeding for many small animals, such as worms and arthropods. Some intestinal nematodes, such as Ancylostomatids, feed on blood extracted from the capillaries of the gut, and about 75 percent of all species of leeches are hematophagous. The spider Evarcha culicivora feeds indirectly on vertebrate blood by specializing on blood-filled female mosquitoes as their preferred prey. Some fish, such as lampreys and candirus; mammals, especially vampire bats; and birds, including the vampire finch, Hood mockingbird, Tristan thrush, and oxpeckers, also practise hematophagy.
This article describes disparities existing between men and women in accessing and receiving care for a stroke. This article also describes factors outside of the health care system which contribute to this disparity.
Thrombus perviousness is an imaging biomarker which is used to estimate clot permeability from CT imaging. It reflects the ability of artery-occluding thrombi to let fluid seep into and through them. The more pervious a thrombus, the more fluid it lets through. Thrombus perviousness can be measured using radiological imaging routinely performed in the clinical management of acute ischemic stroke: CT scans without intravenous contrast combined with CT scans after intravenously administered contrast fluid. Pervious thrombi may let more blood pass through to the ischemic brain tissue, and/or have a larger contact surface and histopathology more sensitive for thrombolytic medication. Thus, patients with pervious thrombi may have less brain tissue damage by stroke. The value of thrombus perviousness in acute ischemic stroke treatment is currently being researched.
A migrainous infarction is a rare type of ischaemic stroke which occurs in correspondence with migraine aura symptoms. Symptoms include headaches, visual disturbances, strange sensations and dysphasia, all of which gradually worsen causing neurological changes which ultimately increase the risk of an ischaemic stroke. Typically, women under the age of 45 who experience migraine with aura (MA) are at the greatest risk for developing migrainous infarction, especially when combined with smoking and use of oral contraceptives.
A cerebroprotectant is a drug that is intended to protect the brain after the onset of acute ischemic stroke. As stroke is the second largest cause of death worldwide and a leading cause of adult disability, over 150 drugs tested in clinical trials to provide cerebroprotection.