Related Research Articles

Cerebrovascular disease includes a variety of medical conditions that affect the blood vessels of the brain and the cerebral circulation. Arteries supplying oxygen and nutrients to the brain are often damaged or deformed in these disorders. The most common presentation of cerebrovascular disease is an ischemic stroke or mini-stroke and sometimes a hemorrhagic stroke. Hypertension is the most important contributing risk factor for stroke and cerebrovascular diseases as it can change the structure of blood vessels and result in atherosclerosis. Atherosclerosis narrows blood vessels in the brain, resulting in decreased cerebral perfusion. Other risk factors that contribute to stroke include smoking and diabetes. Narrowed cerebral arteries can lead to ischemic stroke, but continually elevated blood pressure can also cause tearing of vessels, leading to a hemorrhagic stroke.

Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain. This typically causes impaired nerve function, increased pressure within the skull, and can eventually lead to direct compression of brain tissue and blood vessels. Symptoms vary based on the location and extent of edema and generally include headaches, nausea, vomiting, seizures, drowsiness, visual disturbances, dizziness, and in severe cases, coma and death.
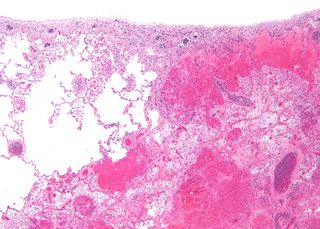
Infarction is tissue death (necrosis) due to inadequate blood supply to the affected area. It may be caused by artery blockages, rupture, mechanical compression, or vasoconstriction. The resulting lesion is referred to as an infarct (from the Latin infarctus, "stuffed into").
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly.

Cerebral hypoxia is a form of hypoxia, specifically involving the brain; when the brain is completely deprived of oxygen, it is called cerebral anoxia. There are four categories of cerebral hypoxia; they are, in order of increasing severity: diffuse cerebral hypoxia (DCH), focal cerebral ischemia, cerebral infarction, and global cerebral ischemia. Prolonged hypoxia induces neuronal cell death via apoptosis, resulting in a hypoxic brain injury.

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.

Intraparenchymal hemorrhage (IPH) is one form of intracerebral bleeding in which there is bleeding within brain parenchyma. The other form is intraventricular hemorrhage (IVH).

Cerebral infarction is the pathologic process that results in an area of necrotic tissue in the brain. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia), most commonly due to thromboembolism, and manifests clinically as ischemic stroke. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.

A watershed stroke is defined as a brain ischemia that is localized to the vulnerable border zones between the tissues supplied by the anterior, posterior and middle cerebral arteries. The actual blood stream blockage/restriction site can be located far away from the infarcts. Watershed locations are those border-zone regions in the brain supplied by the major cerebral arteries where blood supply is decreased. Watershed strokes are a concern because they comprise approximately 10% of all ischemic stroke cases. The watershed zones themselves are particularly susceptible to infarction from global ischemia as the distal nature of the vasculature predisposes these areas to be most sensitive to profound hypoperfusion.
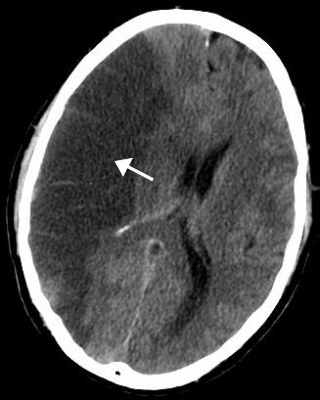
Brain ischemia is a condition in which there is insufficient bloodflow to the brain to meet metabolic demand. This leads to poor oxygen supply or cerebral hypoxia and thus leads to the death of brain tissue or cerebral infarction/ischemic stroke. It is a sub-type of stroke along with subarachnoid hemorrhage and intracerebral hemorrhage.
Animal models of ischemic stroke are procedures inducing cerebral ischemia. The aim is the study of basic processes or potential therapeutic interventions in this disease, and the extension of the pathophysiological knowledge on and/or the improvement of medical treatment of human ischemic stroke. Ischemic stroke has a complex pathophysiology involving the interplay of many different cells and tissues such as neurons, glia, endothelium, and the immune system. These events cannot be mimicked satisfactorily in vitro yet. Thus a large portion of stroke research is conducted on animals.

The leptomeningeal collateral circulation is a network of small blood vessels in the brain that connects branches of the middle, anterior and posterior cerebral arteries, with variation in its precise anatomy between individuals. During a stroke, leptomeningeal collateral vessels allow limited blood flow when other, larger blood vessels provide inadequate blood supply to a part of the brain.
A silent stroke is a stroke that does not have any outward symptoms associated with stroke, and the patient is typically unaware they have suffered a stroke. Despite not causing identifiable symptoms, a silent stroke still causes damage to the brain and places the patient at increased risk for both transient ischemic attack and major stroke in the future. In a broad study in 1998, more than 11 million people were estimated to have experienced a stroke in the United States. Approximately 770,000 of these strokes were symptomatic and 11 million were first-ever silent MRI infarcts or hemorrhages. Silent strokes typically cause lesions which are detected via the use of neuroimaging such as MRI. The risk of silent stroke increases with age but may also affect younger adults. Women appear to be at increased risk for silent stroke, with hypertension and current cigarette smoking being amongst the predisposing factors.

Perfusion MRI or perfusion-weighted imaging (PWI) is perfusion scanning by the use of a particular MRI sequence. The acquired data are then post-processed to obtain perfusion maps with different parameters, such as BV, BF, MTT and TTP.

An MRI sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
Cerebral blood volume is the blood volume in a given amount of brain tissue.
Very low cerebral blood volume (VLCBV) is a measurement of hemorrhagic transformation degree in the tissue surrounding the lesion in strokes. It is counted as one of the penumbral imaging procedures along with less commonly used methods such as diffusion-weighted imaging (DWI). These are used to predict if there is going to be a hemorrhage after the treatment by tPA. In advanced centers, this measurement helps with using tPA beyond the standard time limit without risk of hemorrhage.

Arterial occlusion is a condition involving partial or complete blockage of blood flow through an artery. Arteries are blood vessels that carry oxygenated blood to body tissues. An occlusion of arteries disrupts oxygen and blood supply to tissues, leading to ischemia. Depending on the extent of ischemia, symptoms of arterial occlusion range from simple soreness and pain that can be relieved with rest, to a lack of sensation or paralysis that could require amputation.
A cerebroprotectant is a drug that is intended to protect the brain after the onset of acute ischemic stroke. As stroke is the second largest cause of death worldwide and a leading cause of adult disability, over 150 drugs tested in clinical trials to provide cerebroprotection.

Jean-Claude Baron is an Emeritus Professor of Stroke Medicine at the University of Cambridge. He is also a Fellow of the Academy of Medical Sciences. He has authored around 450 peer-reviewed articles.
References
- 1 2 3 Guadagno J.; Calautti C.; Baron J. (2003). "Progress in imaging stroke: emerging clinical applications". British Medical Bulletin. 65 (1): 145–157. doi: 10.1093/bmb/65.1.145 . PMID 12697622.
- ↑ Fisher M, Ginsberg M (2004). "Current Concepts of the Ischemic Penumbra". Stroke. 32 (11_suppl_1): 2657–2658. doi: 10.1161/01.STR.0000143217.53892.18 .
- 1 2 3 Eng H Lo. (2008). "A New Penumbra: Transitioning from injury into repair after stroke". Nature Medicine . 14 (5): 497–500. doi:10.1038/nm1735. PMID 18463660. S2CID 205385488.
- 1 2 3 4 Hakim (September 1998). "The penumbra: The therapeutic window". Neurology. 51 (3): 44–6. doi:10.1212/wnl.51.3_suppl_3.s44. PMID 9744833. S2CID 44452236.
- 1 2 Chen, Feng (2012). "Magnetic resonance diffusion-perfusion mismatch in acute ischemic stroke: An update". World Journal of Radiology. 4 (3): 63–74. doi:10.4329/wjr.v4.i3.63. ISSN 1949-8470. PMC 3314930 . PMID 22468186.
- ↑ Rowley H (2001). "The four p's of acute stroke imaging: parenchyma, pipes, perfusion, and penumbra". American Journal of Neuroradiology. 22 (4): 599–601. PMC 7976007 . PMID 11290464.
- ↑ Herholz, K. (2000). "Functional Imaging Correlates of Recovery After Stroke in Humans". Journal of Cerebral Blood Flow & Metabolism. 20 (12): 619–631. doi: 10.1097/00004647-200012000-00001 . PMID 11129778.
- ↑ Cserép, Csaba; Pósfai, Balázs; Lénárt, Nikolett; Fekete, Rebeka; László, Zsófia I.; Lele, Zsolt; Orsolits, Barbara; Molnár, Gábor; Heindl, Steffanie; Schwarcz, Anett D.; Ujvári, Katinka; Környei, Zsuzsanna; Tóth, Krisztina; Szabadits, Eszter; Sperlágh, Beáta; Baranyi, Mária; Csiba, László; Hortobágyi, Tibor; Maglóczky, Zsófia; Martinecz, Bernadett; Szabó, Gábor; Erdélyi, Ferenc; Szipőcs, Róbert; Tamkun, Michael M.; Gesierich, Benno; Duering, Marco; Katona, István; Liesz, Arthur; Tamás, Gábor; Dénes, Ádám (31 January 2020). "Microglia monitor and protect neuronal function through specialized somatic purinergic junctions". Science. 367 (6477): 528–537. doi:10.1126/science.aax6752. PMID 31831638. S2CID 209343260.