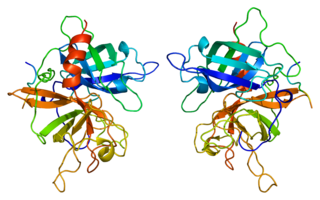A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to stop and prevent further bleeding, but can be harmful in thrombosis, when a clot obstructs blood flow through healthy blood vessels in the circulatory system.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.
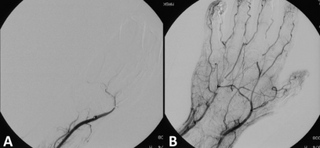
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.
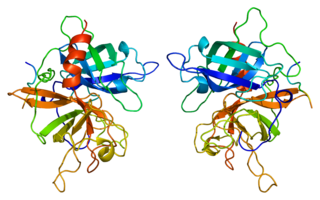
Tissue-type plasminogen activator, short name tPA, is a protein that facilitates the breakdown of blood clots. It acts as an enzyme to convert plasminogen into its active form plasmin, the major enzyme responsible for clot breakdown. It is a serine protease found on endothelial cells lining the blood vessels. Human tPA is encoded by the PLAT gene, and has a molecular weight of ~70 kDa in the single-chain form.

Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.
Streptokinase is a thrombolytic medication activating plasminogen by nonenzymatic mechanism. As a medication it is used to break down clots in some cases of myocardial infarction, pulmonary embolism, and arterial thromboembolism. The type of heart attack it is used in is an ST elevation myocardial infarction (STEMI). It is given by injection into a vein.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.
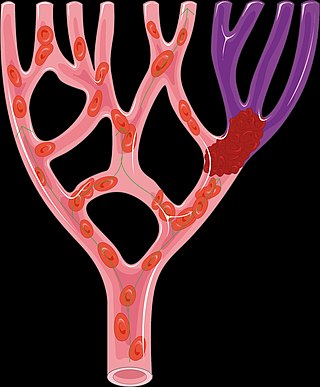
Alteplase, sold under the brand name Activase among others, is a biosynthetic form of human tissue-type plasminogen activator (t-PA). It is a thrombolytic medication used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.
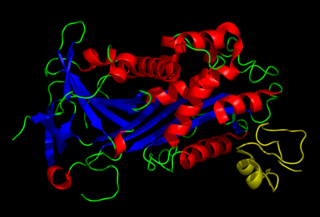
Plasminogen activator inhibitor-1 (PAI-1) also known as endothelial plasminogen activator inhibitor is a protein that in humans is encoded by the SERPINE1 gene. Elevated PAI-1 is a risk factor for thrombosis and atherosclerosis.

Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.

The Urokinase receptor, also known as urokinase plasminogen activator surface receptor (uPAR) or CD87, is a protein encoded in humans by the PLAUR gene. It is a multidomain glycoprotein tethered to the cell membrane with a glycosylphosphotidylinositol (GPI) anchor. uPAR was originally identified as a saturable binding site for urokinase on the cell surface.
Tenecteplase, sold under the trade names TNKase, Metalyse and Elaxim, is an enzyme used as a thrombolytic drug.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.
RTPA or rtPA may refer to:
Proteases are in use, or have been proposed or tried, for a number of purposes related to medicine or surgery. Some preparations involving protease have undergone successful clinical trials and have regulatory authorization; and some further ones have shown apparently useful effects in experimental medical studies. Proteases have also been used by proponents of alternative therapies, or identified in materials of traditional or folk medicine. A serine protease of human origin, activated protein C, was produced in recombinant form and marketed as Drotrecogin alfa and licensed for intensive-care treatment of severe sepsis. It was voluntarily withdrawn by the manufacturer in 2011 after being shown to be ineffective.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon and/or stents to open up the artery. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.

Tiplasinin or tiplaxtinin (PAI-039) is a drug which acts as an inhibitor of the serpin protein plasminogen activator inhibitor-1 (PAI-1), thereby increasing activity of the enzymes tissue plasminogen activator and urokinase, which are involved in the blood clotting cascade. Inhibition of PAI-1 can help to prevent damage to blood vessel walls that occurs as a consequence of chronic high blood pressure, as well as preventing the formation of blood clots that can lead to stroke and heart attack, and potentially also providing a novel treatment mechanism to slow the development of diabetes and obesity. Tiplasinin was unsuccessful in human clinical trials due to an unfavourable risk to benefit ratio and the need for tight dose control to avoid provoking bleeding disorders, however it is still widely used in scientific research and newer drugs sharing the same mechanism of action are likely to be developed for medical use in future.