This article's lead section may need to be rewritten. The reason given is: More than half of the lead is about recent pharmaceuticals, not the natural protein.(April 2024) |
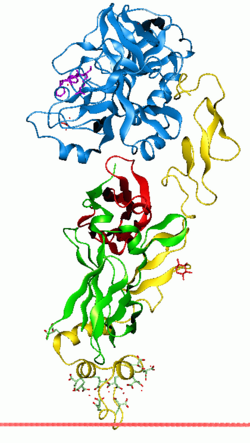
Coagulation factor VII (EC 3.4.21.21, formerly known as proconvertin) is a protein involved in coagulation and, in humans, is encoded by gene F7. It is an enzyme of the serine protease class. Once bound to tissue factor released from damaged tissues, it is converted to factor VIIa (or blood-coagulation factor VIIa, activated blood coagulation factor VII), which in turn activates factor IX and factor X.
Using genetic recombination a recombinant factor VIIa (eptacog alfa) (trade names include NovoSeven) has been approved by the FDA for the control of bleeding in hemophilia. [5] It is sometimes used unlicensed in severe uncontrollable bleeding, although there have been safety concerns. A biosimilar form of recombinant activated factor VII (AryoSeven) is also available, but does not play any considerable role in the market.
In April 2020, the US FDA approved a new rFVIIa product, eptacog beta (SEVENFACT), the first bypassing agent (BPA) approved in more than 2 decades. As an rFVIIa product, eptacog beta works in a complex with tissue factor to activate factor X to Xa, thereby bypassing FVIII and FIX. The activation of Factor X to Xa initiates the coagulation cascade's common pathway, leading to clot formation at the site of hemorrhage. Activated FVII binds to endothelial protein C receptor (EPCR), which enhances hemostasis.14 One study showed that eptacog beta binds to EPCR with 25% to 30% more affinity than eptacog alfa, displacing protein C from EPCR binding sites and downregulating activated protein C generation, contributing to its hemostatic effect.