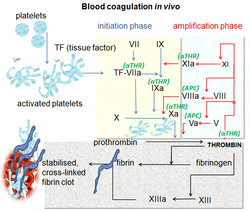
| Number/Name | Synonym(s) | Function | Associated genetic disorders | Type of molecule | Source | Pathway(s) |
|---|
| Factor I | Fibrinogen | Forms fibrin threads in blood clots | | Plasma protein | Liver | Common pathway; converted into fibrin |
| Factor II* | Prothrombin | Its active form (IIa) activates platelets, factors I, V, VII, VIII, XI, XIII, protein C | | Plasma protein | Liver | Common pathway; converted into thrombin |
| Factor III | | Co-factor of factor VIIa, which was formerly known as factor III | | Lipoprotein mixture | Damaged cells and platelets | Extrinsic |
| Factor IV | - Calcium
- Calcium ions
- Ca2+ ions
| Required for coagulation factors to bind to phospholipids, which were formerly known as factor IV | | Inorganic ions in plasma | Diet, platelets, bone matrix | Entire process of coagulation |
| Factor V | - Proaccelerin
- labile factor
- Ac-globulin
| Co-factor of factor X with which it forms the prothrombinase complex | Activated protein C resistance | Plasma protein | Liver, platelets | Extrinsic and intrinsic |
| Factor VI | - Unassigned –
old name of factor Va
(activated form of factor V) - accelerin (formerly)
| N/A | N/A | N/A | |
| Factor VII* | - Proconvertin
- Serum Prothrombin Conversion Accelerator (SPCA)
- Stable factor
| Activates factors IX, X; increases rate of catalytic conversion of prothrombin into thrombin | Congenital factor VII deficiency | Plasma protein | Liver | Extrinsic |
| Factor VIII | - Antihemophilic factor A
- Antihemophilic factor (AHF)
- Antihemophilic globulin (AHG)
| Co-factor of factor IX with which it forms the tenase complex | Hemophilia A | Plasma protein factor | Platelets and endothelial cells | Intrinsic |
| Factor IX* | - Antihemophilic factor B
- Christmas factor
- plasma thromboplastin component (PTC)
| Activates factor X, forms tenase complex with factor VIII | Hemophilia B | Plasma protein | Liver | Intrinsic |
| Factor X* | - Stuart-Prower factor
- Stuart factor
| Activates factor II, forms prothrombinase complex with factor V | Congenital Factor X deficiency | Protein | Liver | Extrinsic and intrinsic |
| Factor XI | - Plasma thromboplastin antecedent (PTA)
- Antihemophilic factor C
| Activates factor IX | Hemophilia C | Plasma protein | Liver | Intrinsic |
| Factor XII | Hageman factor | Activates XI, VII, prekallikrein and plasminogen | Hereditary angioedema type III | Plasma protein | Liver | Intrinsic; initiates clotting in vitro; also activates plasmin |
| Factor XIII | Fibrin-stabilizing factor | Crosslinks fibrin threads | Congenital factor XIIIa/b deficiency | Plasma protein | Liver, platelets | Common pathway; stabilizes fibrin; slows down fibrinolysis |
| Vitamin K | Clotting vitamin | Essential factor to the hepatic gamma-glutamyl carboxylase that adds a carboxyl group to glutamic acid residues on factors II, VII, IX and X, as well as Protein S, Protein C and Protein Z [8] | Vitamin K deficiency | Phytyl-substituted naphthoquinone derivative | Gut microbiota
(e.g. E. coli [9] ),
dietary sources | Extrinsic [10] |
| von Willebrand factor | | Binds to VIII, mediates platelet adhesion | von Willebrand disease | Blood glycoprotein | Blood vessels' endothelia,
bone marrow [11] | |
| Prekallikrein | Fletcher factor | Activates XII and prekallikrein; cleaves HMWK | Prekallikrein/Fletcher factor deficiency |
| Kallikrein | | Activates plasminogen | |
| High-molecular-weight kininogen | | Supports reciprocal activation of factors XII, XI, and prekallikrein | Kininogen deficiency |
| Fibronectin | | Mediates cell adhesion | Glomerulopathy with fibronectin deposits |
| Antithrombin III | | Inhibits factors IIa, Xa, IXa, XIa, and XIIa | Antithrombin III deficiency |
| Heparin cofactor II | | Inhibits factor IIa, cofactor for heparin and dermatan sulfate ("minor antithrombin") | Heparin cofactor II deficiency |
| Protein C | | Inactivates factors Va and VIIIa | Protein C deficiency |
| Protein S | | Cofactor for activated protein C (APC, inactive when bound to C4b-binding protein | Protein S deficiency |
| Protein Z | | Mediates thrombin adhesion to phospholipids and stimulates degradation of factor X by ZPI | Protein Z deficiency |
| Protein Z-related protease inhibitor | ZPI | Degrades factors X (in presence of protein Z) and XI (independently | |
| Plasminogen | | Converts to plasmin, lyses fibrin and other proteins | Plasminogen deficiency type I (ligneous conjunctivitis) |
| α2-Antiplasmin | | Inhibits plasmin | Antiplasmin deficiency |
| α2-Macroglobulin | | Inhibits plasmin, kallikrein, and thrombin | |
| Tissue plasminogen activator | t-PA or TPA | Activates plasminogen | |
| Urokinase | | Activates plasminogen | Quebec platelet disorder |
| Plasminogen activator inhibitor-1 | PAI-1 | Inactivates tPA and urokinase (endothelial PAI | Plasminogen activator inhibitor-1 deficiency |
| Plasminogen activator inhibitor-2 | PAI-2 | Inactivates tPA and urokinase | Plasminogen activator inhibitor-1 deficiency |
| Cancer procoagulant | | Pathological activator of factor X; linked to thrombosis in various cancers [12] | |
| * Vitamin K is required for biosynthesis of these clotting factors [8] |
|---|