 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 8 to 11 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.281 |
| Chemical and physical data | |
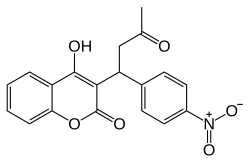
| Formula | C19H15NO6 |
| Molar mass | 353.330 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 196 to 199 °C (385 to 390 °F) |
| |
| |
| (verify) | |
Acenocoumarol is an anticoagulant that functions as a vitamin K antagonist (like warfarin). It is a derivative of coumarin and is generic, so is marketed under many brand names worldwide. [1]