
Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.
PUVA is an ultraviolet light therapy treatment for skin diseases: vitiligo, eczema, psoriasis, graft-versus-host disease, mycosis fungoides, large plaque parapsoriasis, and cutaneous T-cell lymphoma, using the sensitizing effects of the drug psoralen. The psoralen is applied or taken orally to sensitize the skin, then the skin is exposed to UVA.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.

Psoralen is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of Psoralea corylifolia, as well as in the common fig, celery, parsley, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.

The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.

The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. Most of the plant species found to contain furanocoumarins belong to a handful of plant families. The families Apiaceae and Rutaceae include the largest numbers of plant species that contain furanocoumarins. The families Moraceae and Fabaceae include a few widely distributed plant species that contain furanocoumarins.

Bergamottin (5-geranoxypsoralen) is a natural furanocoumarin found in the pulp of pomelos and grapefruits. It is also found in the peel and pulp of the bergamot orange, from which it was first isolated and from which its name is derived.
In enzymology, a trans-cinnamate 4-monooxygenase (EC 1.14.14.91) is an enzyme that catalyzes the chemical reaction

The enzyme 3-dehydroquinate dehydratase (EC 4.2.1.10) catalyzes the chemical reaction

Phytophotodermatitis, also known as berloque dermatitis, margarita photodermatitis, lime disease or lime phytodermatitis is a cutaneous phototoxic inflammatory reaction resulting from contact with a light-sensitizing botanical agent followed by exposure to ultraviolet A (UV-A) light. Symptoms include erythema, edema, blisters, and delayed hyperpigmentation. Heat and moisture tend to exacerbate the reaction.

Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, bergapten has been extracted from 24 species of the genus Heracleum in the family Apiaceae. In the family Rutaceae, various Citrus species contain significant amounts of bergapten, especially the bergamot orange, the micrantha, and certain varieties of lime and bitter orange.
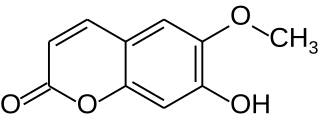
Scopoletin is a coumarin found in the root of plants in the genus Scopolia such as Scopolia carniolica and Scopolia japonica, in chicory, in Artemisia scoparia, in the roots and leaves of stinging nettle, in the passion flower, in Brunfelsia, in Viburnum prunifolium, in Solanum nigrum, in Datura metel, in Mallotus resinosus, or and in Kleinhovia hospita. It can also be found in fenugreek, vinegar, some whiskies or in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarin.
The biosynthesis of phenylpropanoids involves a number of enzymes.

Isopimpinellin is a natural product synthesized by numerous plant species, especially species in the carrot family Apiaceae. The compound can be found in celery, garden angelica, parsnip, fruits and in the rind and pulp of limes. Several studies have looked into the effects of isopimpinellin and other furanocoumarins as anticarcinogens. These studies have shown possible inhibition of 7,12-Dimethylbenz(a)anthracene, which are initiators of skin tumors. Evidence has also been reported that links these compounds to the inhibition of breast cancers.

Umbellic acid is a hydroxycinnamic acid. It is an isomer of caffeic acid.


















