Vitamin K is a family of structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation or for controlling binding of calcium in bones and other tissues. The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor.

An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.

Warfarin, sold under the brand name Coumadin among others, is an anticoagulant medication. While the drug is described as a "blood thinner", it does not reduce viscosity but rather prevents blood clots (thrombus) from forming (coagulating). Accordingly, it is commonly used to prevent deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Warfarin may sometimes be prescribed following ST-segment elevation myocardial infarctions (STEMI) and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously. It is a vitamin K antagonist.
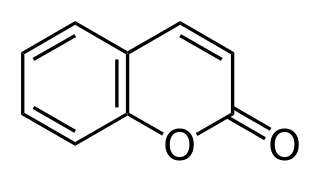
Coumarin or 2H-chromen-2-one is an aromatic organic chemical compound with formula C9H6O2. Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by an unsaturated lactone ring −(CH)=(CH)−(C=O)−O−, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It belongs to the benzopyrone chemical class and considered as a lactone.

Warfarin-induced skin necrosis is a condition in which skin and subcutaneous tissue necrosis occurs due to acquired protein C deficiency following treatment with anti-vitamin K anticoagulants.

Coumatetralyl is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type used as a rodenticide.
Karl Paul Gerhard Link was an American biochemist best known for his discovery of the anticoagulant warfarin.

Phenprocoumon is a long-acting anticoagulant to be taken by mouth, and a coumarin derivative. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.

Dicoumarol (INN) or dicumarol (USAN) is a naturally occurring anticoagulant drug that depletes stores of vitamin K. It is also used in biochemical experiments as an inhibitor of reductases.

Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger pests such as possums.
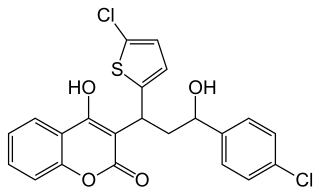
Tioclomarol is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type. It is a second generation drug, used as a rodenticide that is effective for the control of rodents that are resistant to this class of drugs.

Diphenadione is a vitamin K antagonist that has anticoagulant effects and is used as a rodenticide against rats, mice, voles, ground squirrels and other rodents. The chemical compound is an anti-coagulant with active half-life longer than warfarin and other synthetic 1,3-indandione anticoagulants.

Bromadiolone is a potent anticoagulant rodenticide. It is a second-generation 4-hydroxycoumarin derivative and vitamin K antagonist, often called a "super-warfarin" for its added potency and tendency to accumulate in the liver of the poisoned organism. When first introduced to the UK market in 1980, it was effective against rodent populations that had become resistant to first generation anticoagulants.
In enzymology, a vitamin-K-epoxide reductase (warfarin-sensitive) is an enzyme that catalyzes the chemical reaction

The human gene VKORC1 encodes for the enzyme, Vitamin K epOxide Reductase Complex (VKORC) subunit 1. This enzymatic protein complex is responsible for reducing vitamin K 2,3-epoxide to its active form, which is important for effective clotting (coagulation). In humans, mutations in this gene can be associated with deficiencies in vitamin-K-dependent clotting factors.

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonize the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K. Vitamin K antagonists (VKAs) have been the mainstay of anticoagulation therapy for more than 50 years.

Chlorophacinone is a first-generation anticoagulant rodenticide. The mechanism of action results in internal bleeding due to non-functional clotting factors. It was used as a toxin to control rodent populations. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

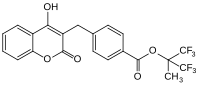
4-Hydroxycoumarin is a coumarin derivative with a hydroxy group at the 4-position.
Four drugs from the class of direct Xa inhibitors are marketed worldwide. Rivaroxaban (Xarelto) was the first approved FXa inhibitor to become commercially available in Europe and Canada in 2008. The second one was apixaban (Eliquis), approved in Europe in 2011 and in the United States in 2012. The third one edoxaban was approved in Japan in 2011 and in Europe and the US in 2015. Betrixaban (Bevyxxa) was approved in the US in 2017.

Coumarin derivatives are derivatives of coumarin and are considered phenylpropanoids. Among the most important derivatives are the 4-hydroxycoumarins, which exhibit anticoagulant properties, a characteristic not present for coumarin itself.