Warfarin, sold under the brand name Coumadin among others, is an anticoagulant medication. While the drug is described as a "blood thinner", it does not reduce viscosity but rather prevents blood clots (thrombus) from forming (coagulating). Accordingly, it is commonly used to prevent deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Warfarin may sometimes be prescribed following ST-segment elevation myocardial infarctions (STEMI) and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously. It is a vitamin K antagonist.
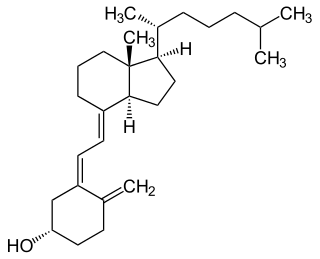
Cholecalciferol, also known as vitamin D3 or colecalciferol, is a type of vitamin D that is produced by the skin when exposed to UVB light; it is found in certain foods and can be taken as a dietary supplement.

Rodenticides are chemicals made and sold for the purpose of killing rodents. While commonly referred to as "rat poison", rodenticides are also used to kill mice, woodchucks, chipmunks, porcupines, nutria, beavers, and voles. Despite the crucial roles that rodents play in nature, there are times when they need to be controlled.
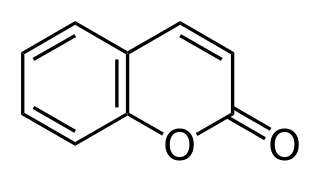
Coumarin or 2H-chromen-2-one is an aromatic organic chemical compound with formula C9H6O2. Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by an unsaturated lactone ring −(CH)=(CH)−(C=O)−O−, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It belongs to the benzopyrone chemical class and considered as a lactone.

Aminopterin, the 4–amino derivative of folic acid, is an antineoplastic drug with immunosuppressive properties often used in chemotherapy. Aminopterin is a synthetic derivative of pterin. Aminopterin works as an enzyme inhibitor by competing for the folate binding site of the enzyme dihydrofolate reductase. Its binding affinity for dihydrofolate reductase effectively blocks tetrahydrofolate synthesis. This results in the depletion of nucleotide precursors and inhibition of DNA, RNA, and protein synthesis.

Bromethalin is a neurotoxic rodenticide that damages the central nervous system.

Coumatetralyl is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type used as a rodenticide.

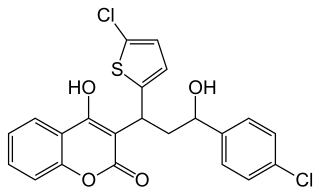
4-Hydroxycoumarins are a class of vitamin K antagonist (VKA) anticoagulant drug molecules. Chemically, they are derived from coumarin by adding a hydroxy group at the 4 position to obtain 4-hydroxycoumarin, then adding a large aromatic substituent at the 3-position. The large 3-position substituent is required for anticoagulant activity.

Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger pests such as possums.
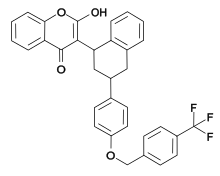
Tioclomarol is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type. It is a second generation drug, used as a rodenticide that is effective for the control of rodents that are resistant to this class of drugs.

Diphenadione is a vitamin K antagonist that has anticoagulant effects and is used as a rodenticide against rats, mice, voles, ground squirrels and other rodents. The chemical compound is an anti-coagulant with active half-life longer than warfarin and other synthetic 1,3-indandione anticoagulants.

Bromadiolone is a potent anticoagulant rodenticide. It is a second-generation 4-hydroxycoumarin derivative and vitamin K antagonist, often called a "super-warfarin" for its added potency and tendency to accumulate in the liver of the poisoned organism. When first introduced to the UK market in 1980, it was effective against rodent populations that had become resistant to first generation anticoagulants.

Methiocarb is a carbamate pesticide which is used as an insecticide, bird repellent, acaricide and molluscicide since the 1960s. Methiocarb has contact and stomach action on mites and neurotoxic effects on molluscs. Seeds treated with methiocarb also affect birds. Other names for methiocarb are mesurol and mercaptodimethur.

Difenacoum is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type. It has anticoagulant effects and is used commercially as a rodenticide. It was first introduced in 1976 and first registered in the USA in 2007.

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonize the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K. Vitamin K antagonists (VKAs) have been the mainstay of anticoagulation therapy for more than 50 years.

Chlorophacinone is a first-generation anticoagulant rodenticide. The mechanism of action results in internal bleeding due to non-functional clotting factors. It was used as a toxin to control rodent populations. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
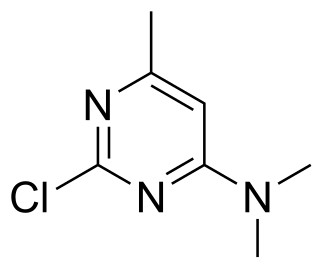
Crimidine is a convulsant poison used as a rodenticide. Crimidine was originally known by its product name, Castrix. It was originally produced in the 1940s by the conglomerate, IG Farben. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities. It is also no longer used in the United States as a rodenticide, but is still used to this day in other countries.

Norbormide is a toxic compound used as a rodenticide. It has several mechanisms of action, acting as a vasoconstrictor and calcium channel blocker, but is selectively toxic to rats and has relatively low toxicity to other species, due to a species specific action of opening the permeability transition pores in rat mitochondria.

d-CON is an America brand of rodent control products, which is distributed and owned in the United States by the UK-based consumer goods company Reckitt.

Tebufenpyrad is an insecticide and acaricide widely used in greenhouses. It is a white solid chemical with a slight aromatic smell. It is soluble in water and also in organic solvents.