Related Research Articles
Alexander Neville was a late medieval prelate who served as Archbishop of York from 1374 to 1388.

Brodifacoum is a highly lethal 4-hydroxycoumarin vitamin K antagonist anticoagulant poison. In recent years, it has become one of the world's most widely used pesticides. It is typically used as a rodenticide, but is also used to control larger pests such as possum.

Transient receptor potential cation channel, subfamily A, member 1, also known as transient receptor potential ankyrin 1, TRPA1, or The Wasabi Receptor, is a protein that in humans is encoded by the TRPA1 gene.

2-Arachidonyl glyceryl ether is a putative endocannabinoid discovered by Lumír Hanuš and colleagues at the Hebrew University of Jerusalem, Israel. It is an ether formed from the alcohol analog of arachidonic acid and glycerol. Its isolation from porcine brain and its structural elucidation and synthesis were described in 2001.
The alpha-4 beta-2 nicotinic receptor, also known as the α4β2 receptor, is a type of nicotinic acetylcholine receptor implicated in learning, consisting of α4 and β2 subunits. It is located in the brain, where activation yields post- and presynaptic excitation, mainly by increased Na+ and K+ permeability.

NAD-dependent deacetylase sirtuin 7 is an enzyme that in humans is encoded by the SIRT7 gene. SIRT7 is member of the mammalian sirtuin family of proteins, which are homologs to the yeast Sir2 protein.
1374 Isora, provisional designation 1935 UA, is a stony asteroid and eccentric Mars-crosser from the innermost regions of the asteroid belt, approximately 5 kilometers in diameter. It was discovered on 21 October 1935, by Belgian astronomer Eugène Delporte at Uccle Observatory in Belgium.
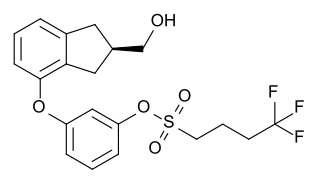
Originally synthesized by chemist Wayne E. Kenney, BAY 38-7271 (KN 38-7271) is a drug which is a cannabinoid receptor agonist developed by Bayer AG. It has analgesic and neuroprotective effects and is used in scientific research, with proposed uses in the treatment of traumatic brain injury. It is a full agonist with around the same potency as CP 55,940 in animal studies, and has fairly high affinity for both CB1 and CB2 receptors, with Ki values of 2.91nM at CB1 and 4.24nM at CB2. It has been licensed to KeyNeurotek Pharmaceuticals for clinical development, and was in Phase II trials in 2008 but its development appears to have stopped.
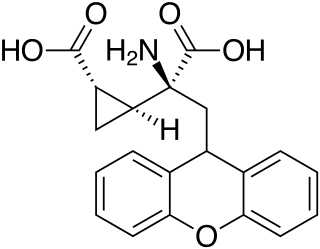
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonise the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K.

AS-8112 is a synthetic compound that acts as a selective antagonist at the dopamine receptor subtypes D2 and D3, and the serotonin receptor 5-HT3. It has potent antiemetic effects in animal studies and has been investigated for potential medical use.
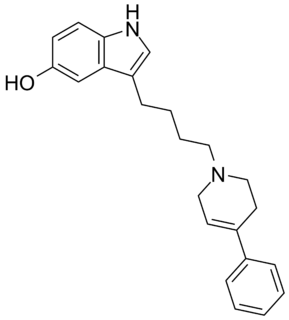
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.
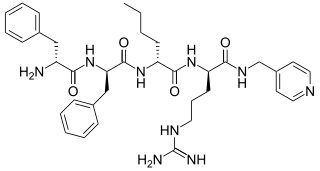
CR665 (H-D-Phe-D-Phe-D-Nle-D-Arg-NH-4-Picolyl), also known by the previous developmental code names FE-200665 and JNJ-38488502, is an all D-amino acid peptide that acts as a peripherally restricted κ-opioid receptor agonist. The selectivity for FE 200665 is 1/16,900/84,600 for the human κ, μ, and δ opioid receptors, respectively. The dose of FE 200665 required to produce motor impairment was 548 times higher than the dose required for antinociceptive activity. It is being developed for use by Cara Therapeutics under the code name CR665.
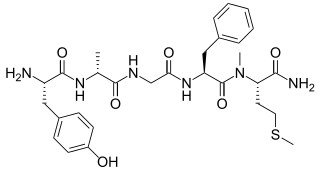
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.
PF-04457845 is a potent and exquisitely selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 7.2nM, and both analgesic and antiinflammatory effects in animal studies comparable to naproxen.

Donitriptan (INN) is a triptan drug which was investigated as an antimigraine agent but ultimately was never marketed. It acts as a high-affinity, high-efficacy/near-full agonist of the 5-HT1B and 5-HT1D receptors, and is among the most potent of the triptan series of drugs. Donitriptan was being developed in France by bioMérieux-Pierre Fabre and made it to phase II clinical trials in Europe before development was discontinued.

BTRX-246040, also known as LY-2940094, is a potent and selective nociceptin receptor antagonist which is under development by BlackThorn Therapeutics and Eli Lilly for the treatment of major depressive disorder (MDD). It has demonstrated proof-of-concept clinical efficacy for depression. As of 2017, it is in phase II clinical trials for the treatment of MDD. It was also under investigation for the treatment of alcoholism, and similarly reached phase II clinical studies for this indication, but development was discontinued.
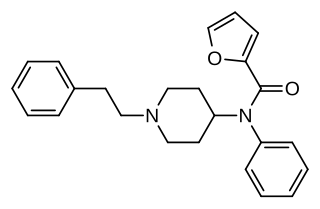
Furanylfentanyl (Fu-F) is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. It has an ED50 value of 0.02 mg/kg in mice. This makes it approximately one fifth as potent as fentanyl.

SC-8109 is a steroidal antimineralocorticoid of the spirolactone group which was never marketed. It is a potent antagonist of the mineralocorticoid receptor and is more potent than the related drug SC-5233. However, SC-8109 was found to have relatively low oral bioavailability and potency, though it nonetheless produced a mild diuretic effect in patients with congestive heart failure. Spironolactone, another spirolactone, followed and had both good oral bioavailability and potency, and was the first antimineralocorticoid to be marketed.
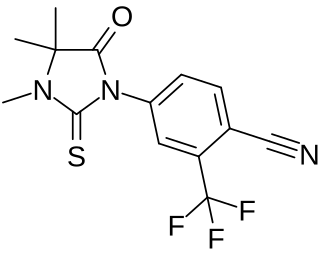
RU-56187 is a nonsteroidal antiandrogen which was never marketed. It shows 92% of the affinity of testosterone for the androgen receptor and negligible affinity for other steroid hormone receptors. The medication is a silent antagonist of the androgen receptor. RU-56187 is 3- to 10-fold more potent as an antiandrogen than bicalutamide or nilutamide in animals. Both RU-56187 and RU-58841 appear to be prodrugs of cyanonilutamide (RU-56279) in vivo in animals.
References
- ↑ Feinstein, D. L; Akpa, B. S; Ayee, M. A; Boullerne, A. I; Braun, D; Brodsky, S. V; Gidalevitz, D; Hauck, Z; Kalinin, S; Kowal, K; Kuzmenko, I; Lis, K; Marangoni, N; Martynowycz, M. W; Rubinstein, I; Van Breemen, R; Ware, K; Weinberg, G (2016). "The emerging threat of superwarfarins: history, detection, mechanisms, and countermeasures". Annals of the New York Academy of Sciences. 1374 (1): 111–22. Bibcode:2016NYASA1374..111F. doi:10.1111/nyas.13085. PMC 4940222 . PMID 27244102.