
Ibotenic acid or (S)-2-amino-2-(3-hydroxyisoxazol-5-yl)acetic acid, also referred to as ibotenate, is a chemical compound and psychoactive drug which occurs naturally in Amanita muscaria and related species of mushrooms typically found in the temperate and boreal regions of the northern hemisphere. It is a prodrug of muscimol, broken down by the liver to that much more stable compound. It is a conformationally-restricted analogue of the neurotransmitter glutamate, and due to its structural similarity to this neurotransmitter, acts as a non-selective glutamate receptor agonist. Because of this, ibotenic acid can be a powerful neurotoxin in high doses, and is employed as a "brain-lesioning agent" through cranial injections in scientific research. The neurotoxic effects appear to be dose-related and risks are unclear through consumption of ibotenic-acid containing fungi, although thought to be negligible in small doses.

Muscimol is one of the principal psychoactive constituents of Amanita muscaria and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptor and displays sedative-hypnotic, depressant and hallucinogenic psychoactivity. This colorless or white solid is classified as an isoxazole.
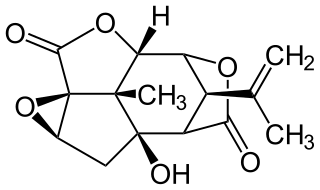
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison). A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.
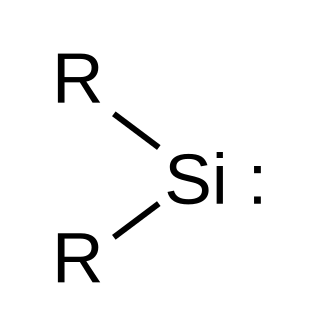
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.

Cicutoxin is a naturally-occurring poisonous chemical compound produced by several plants from the family Apiaceae including water hemlock (Cicuta species) and water dropwort (Oenanthe crocata). The compound contains polyene, polyyne, and alcohol functional groups and is a structural isomer of oenanthotoxin, also found in water dropwort. Both of these belong to the C17-polyacetylenes chemical class.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.
Isonipecotic acid is a heterocyclic compound which acts as a GABAA receptor partial agonist.

Gaboxadol, also known as 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP), is a conformationally constrained derivative of the alkaloid muscimol that was first synthesized in 1977 by the Danish chemist Poul Krogsgaard-Larsen. In the early 1980s gaboxadol was the subject of a series of pilot studies that tested its efficacy as an analgesic and anxiolytic, as well as a treatment for tardive dyskinesia, Huntington's disease, Alzheimer's disease, and spasticity. It was not until 1996 that researchers attempted to harness gaboxadol's frequently reported sedative "adverse effect" for the treatment of insomnia, resulting in a series of clinical trials sponsored by Lundbeck and Merck. In March, 2007, Merck and Lundbeck cancelled work on the drug, citing safety concerns and the failure of an efficacy trial. It acts on the GABA system, but in a different way from benzodiazepines, Z-Drugs, and barbiturates. Lundbeck states that gaboxadol also increases deep sleep. Unlike benzodiazepines, gaboxadol does not demonstrate reinforcement in mice or baboons despite activation of dopaminergic neurons in the ventral tegmental area.

Etaqualone is a quinazolinone-class GABAergic and is an analogue of methaqualone that was developed in the 1960s and marketed mainly in France and some other European countries. It has sedative, hypnotic, muscle relaxant and central nervous system depressant properties resulting from its agonist activity at the β-subtype of the GABAA receptor, and was used for the treatment of insomnia.

Magnolol is an organic compound that is classified as lignan. It is a bioactive compound found in the bark of the Houpu magnolia and in M. grandiflora. The compound exists at the level of a few percent in the bark of species of magnolia, the extracts of which have been used in traditional Chinese and Japanese medicine. In addition to magnolol, related lignans occur in the extracts including honokiol, which is an isomer of magnolol.

Isovaleramide is an organic compound with the formula (CH3)2CHCH2C(O)NH2. The amide derived from isovaleric acid, it is a colourless solid.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

Gamma-aminobutyric acid (GABA) A receptor, alpha 5, also known as GABRA5, is a protein which in humans is encoded by the GABRA5 gene.

SH-053-R-CH3-2′F is a drug used in scientific research which is a benzodiazepine derivative. It produces some of the same effects as other benzodiazepines, but is much more subtype-selective than most other drugs of this class, having high selectivity, binding affinity and efficacy at the α5 subtype of the GABAA receptor. This gives much tighter control of the effects produced, and so while SH-053-R-CH3-2′F retains sedative and anxiolytic effects, it does not cause ataxia at moderate doses. SH-053-R-CH3-2′F also blocks the nootropic effects of the α5-selective inverse agonist PWZ-029, so amnesia is also a likely side effect.

AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

A thienotriazolodiazepine is a heterocyclic compound containing a diazepine ring fused to thiophene and triazole rings. Thienotriazolodiazepine forms the central core of several pharmaceutical drugs including:
1-(4-Chlorophenyl)silatrane is an extremely toxic organosilicon compound which was developed by M&T Chemicals as a single-dose rodenticide. It was never registered as rodenticide, except for experimental use. 1-(4-Chlorophenyl)silatrane was one of the chemicals studied in the Project Coast.

SH-I-048A (SH-i-048A) is a benzodiazepine derivative related in structure to compounds such as flubromazepam and meclonazepam. SH-I-048A is described as a non subtype selective superagonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 0.77 nM at the α1 subtype, 0.17 nM at α2, 0.38 nM at α3 and 0.11 nM at α5. It has been used to study the functional differences between the different subtypes of the GABAA receptor.

GL-II-73 (GL-ii-073) is a benzodiazepine derivative related in chemical structure to compounds such as midazolam and adinazolam. It is described as an α5 preferring positive allosteric modulator of the benzodiazepine site of GABAA receptors, with weaker activity at α2 and α3 and no significant affinity for the α1 subtype. In animal tests it was found to produce effects consistent with antidepressant, anxiolytic and nootropic actions.

















