Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.

Primidone, sold under various brand names, is a medication used to treat seizures including partial and generalized seizures. It may also be used for essential tremors. The dose may be based on levels measured in the blood. It is taken by mouth.
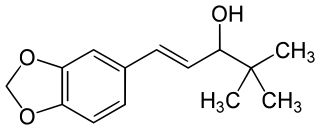
Stiripentol is an anticonvulsant drug used in the treatment of epilepsy. It is approved for the treatment of Dravet syndrome, an epilepsy syndrome. It is unrelated to other anticonvulsants and belongs to the group of aromatic allylic alcohols.
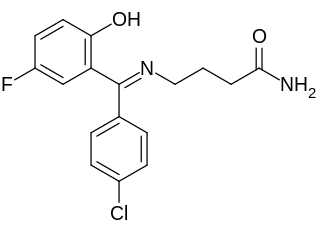
Progabide is an analogue and prodrug of γ-aminobutyric acid (GABA) used in the treatment of epilepsy. Via conversion into GABA, progabide behaves as an agonist of the GABAA, GABAB, and GABAA-ρ receptors.

3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is an organic compound with the formula (CH3)2CHCH2CO2H. It is sometimes classified as a fatty acid. It is a colourless liquid that is sparingly soluble in water, but highly soluble in most common organic solvents. 3-methylbutanoic acid can be found in many common house items, such as cheese, soy milk, and apple juice. The compound occurs naturally.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Etiocholanolone, also known as 5β-androsterone, as well as 3α-hydroxy-5β-androstan-17-one or etiocholan-3α-ol-17-one, is an etiocholane (5β-androstane) steroid as well as an endogenous 17-ketosteroid that is produced from the metabolism of testosterone. It causes fever, immunostimulation, and leukocytosis, and is used to evaluate adrenal cortex function, bone marrow performance, and in neoplastic disease to stimulate the immune system. Etiocholanolone is also known to be an inhibitory androstane neurosteroid, acting as a positive allosteric modulator of the GABAA receptor, and possesses anticonvulsant effects. The unnatural enantiomer of etiocholanolone is more potent as a positive allosteric modulator of GABAA receptors and as an anticonvulsant than the natural form.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce sedative effects. Furthermore, they lead to changes in gene transcription.

Methylpentynol is a tertiary hexanol with hypnotic/sedative and anticonvulsant effects and an exceptionally low therapeutic index. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of insomnia, but its use was quickly phased out in response to newer drugs with far more favorable safety profiles.

Imidazenil is an experimental anxiolytic drug which is derived from the benzodiazepine family, and is most closely related to other imidazobenzodiazepines such as midazolam, flumazenil, and bretazenil.

Antifolates are a class of antimetabolite medications that antagonise (that is, block) the actions of folic acid (vitamin B9). Folic acid's primary function in the body is as a cofactor to various methyltransferases involved in serine, methionine, thymidine and purine biosynthesis. Consequently, antifolates inhibit cell division, DNA/RNA synthesis and repair and protein synthesis. Some such as proguanil, pyrimethamine and trimethoprim selectively inhibit folate's actions in microbial organisms such as bacteria, protozoa and fungi. The majority of antifolates work by inhibiting dihydrofolate reductase (DHFR).

SL651498 is an anxiolytic and anticonvulsant drug used in scientific research, with a chemical structure most closely related to β-carboline derivatives such as abecarnil and gedocarnil. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

TPA-023 (MK-0777) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a subtype-selective, mixed agonist-antagonist at GABAA receptors, which acts as a partial agonist at the α2 and α3 subtypes, but as a silent antagonist at α1 and α5 subtypes. It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.
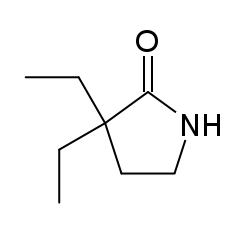
3,3-Diethyl-2-pyrrolidinone (DEABL) is an anticonvulsant drug most closely related to pyrithyldione and gabapentin. It was found to extend lifespan in the nematode worms Caenorhabditis elegans.
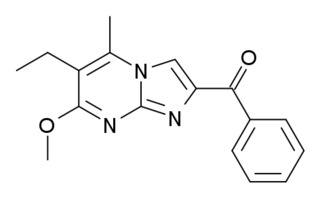
Divaplon (RU-32698) is a nonbenzodiazepine, anxiolytic and anticonvulsant drug from the pyrazolopyrimidine family of drugs. It acts as a partial agonist at the "benzodiazepine site" of the GABAA receptor in the brain.
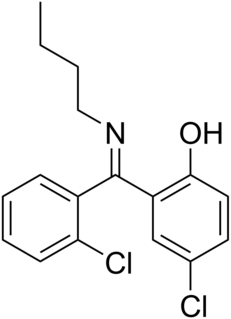
Fengabine (SL-79,229) is a drug which was investigated as an antidepressant but was never marketed. Its mechanism of action is unknown, but its antidepressant effects are reversed by GABAA receptor antagonists like bicuculline and it has hence been labeled as GABAergic; however, it does not actually bind to GABA receptors, nor does it inhibit GABA-T. In clinical trials, fengabine's efficacy was comparable to that of the tricyclic antidepressants, but with a more rapid onset of action and much less side effects. Notably, fengabine lacks any sedative effects.
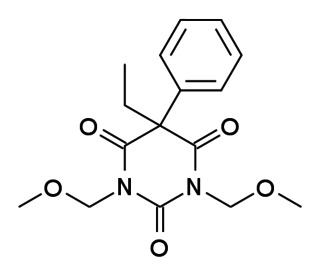
Eterobarb (Antilon) is a barbiturate derivative. It has mainly anticonvulsant action with less sedative effects than the closely related compound phenobarbital. It saw reasonable success in clinical trials, but is not in widespread medical use.

Etazepine (INN) is an anticonvulsant with a tricyclic structure which is related to the benzodiazepines, but was never marketed. It appears to exert its effects via acting through the GABAergic system.

SL-164 is an analogue of methaqualone developed in the late 1960s by a team at Sumitomo. SL-164 has similar sedative, hypnotic and anticonvulsant properties to the parent compound, but was never marketed for clinical use.

A GABA analogue is a compound which is an analogue or derivative of the neurotransmitter gamma-Aminobutyric acid (GABA).


















