| |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
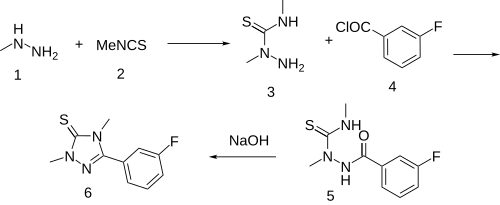
| Formula | C10H10FN3S |
| Molar mass | 223.27 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Suritozole (MDL 26,479) is an investigational cognition enhancer. It acts as a partial inverse agonist at the benzodiazepine receptor site on the GABAA ion channel complex, but does not have either anxiogenic or convulsant effects, unlike other BZD inverse agonists such as DMCM. [1] It was investigated for the treatment of depression and Alzheimer's disease in the 90s, [2] but clinical development seems to have been discontinued.