
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Disulfur decafluoride is a chemical compound with the formula S2F10. It was discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. The two sulfur atoms are connected by a single bond. In the S2F10 molecule, the oxidation state of each sulfur atoms is +5, but their valency is 6. S2F10 is highly toxic, with toxicity four times that of phosgene.

The avermectins are a series of drugs and pesticides used to treat parasitic worm infestations and to reduce insect pests. They are a group of 16-membered macrocyclic lactone derivatives with potent anthelmintic and insecticidal properties. These naturally occurring compounds are generated as fermentation products by Streptomyces avermitilis, a soil actinomycete. Eight different avermectins were isolated in four pairs of homologue compounds, with a major (a-component) and minor (b-component) component usually in ratios of 80:20 to 90:10. Avermectin B1, a mixture of B1a and B1b, is the drug and pesticide abamectin. Other anthelmintics derived from the avermectins include ivermectin, selamectin, doramectin, eprinomectin.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

Sulfuryl chloride fluoride is a chemical compound with the formula SO2ClF. It is a colorless, easily condensed gas. It is a tetrahedral molecule.

Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine.
Mercury(II) fluoride has the molecular formula HgF2 as a chemical compound of one atom of mercury with 2 atoms of fluorine.

Transmembrane activator and CAML interactor (TACI), also known as tumor necrosis factor receptor superfamily member 13B (TNFRSF13B) is a protein that in humans is encoded by the TNFRSF13B gene.

Flurothyl (Indoklon) is a volatile liquid drug from the halogenated ether family, related to inhaled anaesthetic agents such as diethyl ether, but having the opposite effects, acting as a stimulant and convulsant. A clear and stable liquid, it has a mild ethereal odor whose vapors are non-flammable. It is excreted from the body by the lungs in an unchanged state.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.
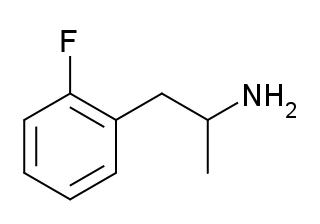
2-Fluoroamphetamine (2-FA) is a stimulant drug from the amphetamine family which has been sold as a designer drug. 2-Fluoroamphetamine differs from 3- and 4-fluoroamphetamine in the position of the fluorine atom on the aromatic ring, making them positional isomers of one another. The replacement of a hydrogen atom with a fluorine atom in certain compounds to facilitate passage through the blood–brain barrier, as is desirable in central nervous system pharmaceutical agents, is a common practice due to the corresponding increase in lipophilicity granted by this substitution.

Ro5-4864 (4'-chlorodiazepam) is a drug which is a benzodiazepine derivative of diazepam. However unlike most benzodiazepine derivatives, Ro5-4864 lacks affinity for GABAA receptors and lacks typical benzodiazepine effects, instead being sedative yet also convulsant and anxiogenic in effects. Ro5-4864 was found to be a potent ligand for the "peripheral benzodiazepine receptor", later renamed to mitochondrial translocator protein 18kDa (TSPO). Despite its convulsant effects, at lower doses Ro5-4864 has proved to be neuroprotective and has become widely used for research into the role of the TSPO protein in neurotoxicity. In vitro studies and rodent models also suggest the possibility of analgesic, antidepressant, cardioprotective, and anti-cancer effects.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

Alstonine is a pentacyclic alkaloid and putative antipsychotic constituent of various plant species including Alstonia boonei, Catharanthus roseus, Picralima nitida, Rauwolfia caffra and Rauwolfia vomitoria. In preclinical studies alstonine attenuates MK-801-induced hyperlocomotion, working memory deficit and social withdrawal. It also possesses anxiolytic-like effects in preclinical studies, attenuates amphetamine-induced lethality and stereotypy as well as apomorphine-induced stereotypy, and attenuates haloperidol-induced catalepsy. These effects appear to be mediated by stimulation of the 5-HT2C receptor. In addition, alstonine, similarly to clozapine, indirectly inhibits the reuptake of glutamate in hippocampal slices. Unlike clozapine however, the effect of which is abolished by the D2 receptor agonist apomorphine, alstonine requires 5-HT2A and 5-HT2C receptors to produce this effect, as it is abolished by antagonists of these receptors. Also unlike clozapine, alstonine lacks pro-convulsant activity in mice.
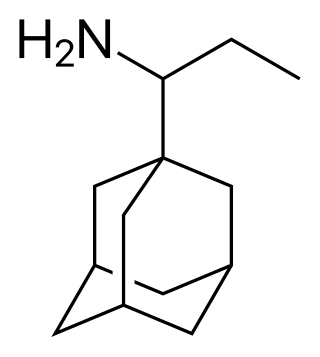
Adapromine is an antiviral drug of the adamantane group related to amantadine (1-aminoadamantane), rimantadine, and memantine (1-amino-3,5-dimethyladamantane) that is marketed in Russia for the treatment and prevention of influenza. It is an alkyl analogue of rimantadine and is similar to rimantadine in its antiviral activity but possesses a broader spectrum of action, being effective against influenza viruses of both type A and B. Strains of type A influenza virus with resistance to adapromine and rimantadine and the related drug deitiforine were encountered in Mongolia and the Soviet Union in the 1980s.

Difluorophosphate or difluorodioxophosphate or phosphorodifluoridate is an anion with formula PO2F−2. It has a single negative charge and resembles perchlorate and monofluorosulfonate in shape and compounds. These ions are isoelectronic, along with tetrafluoroaluminate, phosphate, orthosilicate, and sulfate. It forms a series of compounds. The ion is toxic to mammals as it causes blockage to iodine uptake in the thyroid. However it is degraded in the body over several hours.
The fluorosulfates or fluorosulfonates are a set of salts of fluorosulfuric acid with an ion formula SO3F−. The fluorosulfate anion can be treated as though it were a hydrogen sulfate anion with hydroxyl substituted by fluorine. The fluorosulfate ion has a low propensity to form complexes with metal cations. Since fluorine is similar in size to oxygen, the fluorosulfate ion is roughly tetrahedral and forms salts similar to those of the perchlorate ion. It is isoelectronic with sulfate, SO4−2. When an organic group is substituted for the anions, organic fluorosulfonates are formed.
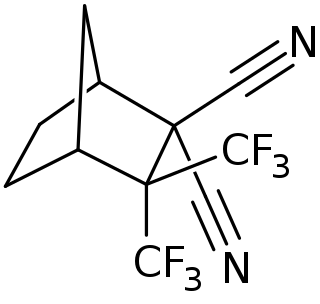
BIDN is a GABA receptor antagonist and convulsant.
1-(4-Chlorophenyl)silatrane is an extremely toxic organosilicon compound which was developed by M&T Chemicals as a single-dose rodenticide. It was never registered as rodenticide, except for experimental use. 1-(4-Chlorophenyl)silatrane was one of the chemicals studied in the Project Coast.

2,2,3,3-Tetrafluoropropyl trifluoromethyl ether is a fluorinated ether with convulsant action.
















