
GABA is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.

Neurotoxins are toxins that are destructive to nerve tissue. Neurotoxins are an extensive class of exogenous chemical neurological insults that can adversely affect function in both developing and mature nervous tissue. The term can also be used to classify endogenous compounds, which, when abnormally contacted, can prove neurologically toxic. Though neurotoxins are often neurologically destructive, their ability to specifically target neural components is important in the study of nervous systems. Common examples of neurotoxins include lead, ethanol, glutamate, nitric oxide, botulinum toxin, tetanus toxin, and tetrodotoxin. Some substances such as nitric oxide and glutamate are in fact essential for proper function of the body and only exert neurotoxic effects at excessive concentrations.

Clonazepam, sold under the brand name Klonopin among others, is a medication used to prevent and treat anxiety disorders, seizures, bipolar mania, agitation associated with psychosis, obsessive–compulsive disorder, and akathisia. It is a long-acting tranquilizer of the benzodiazepine class. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. It is typically taken orally but is also used intravenously. Effects begin within one hour and last between eight and twelve hours in adults.

The GABA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), the chief inhibitory compound in the mature vertebrate central nervous system. There are two classes of GABA receptors: GABAA and GABAB. GABAA receptors are ligand-gated ion channels ; whereas GABAB receptors are G protein-coupled receptors, also called metabotropic receptors.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

Dizocilpine (INN), also known as MK-801, is a pore blocker of the NMDA receptor, a glutamate receptor, discovered by a team at Merck in 1982. Glutamate is the brain's primary excitatory neurotransmitter. The channel is normally blocked with a magnesium ion and requires depolarization of the neuron to remove the magnesium and allow the glutamate to open the channel, causing an influx of calcium, which then leads to subsequent depolarization. Dizocilpine binds inside the ion channel of the receptor at several of PCP's binding sites thus preventing the flow of ions, including calcium (Ca2+), through the channel. Dizocilpine blocks NMDA receptors in a use- and voltage-dependent manner, since the channel must open for the drug to bind inside it. The drug acts as a potent anti-convulsant and probably has dissociative anesthetic properties, but it is not used clinically for this purpose because of the discovery of brain lesions, called Olney's lesions (see below), in laboratory rats. Dizocilpine is also associated with a number of negative side effects, including cognitive disruption and psychotic-spectrum reactions. It inhibits the induction of long term potentiation and has been found to impair the acquisition of difficult, but not easy, learning tasks in rats and primates. Because of these effects of dizocilpine, the NMDA receptor pore blocker ketamine is used instead as a dissociative anesthetic in human medical procedures. While ketamine may also trigger temporary psychosis in certain individuals, its short half-life and lower potency make it a much safer clinical option. However, dizocilpine is the most frequently used uncompetitive NMDA receptor antagonist in animal models to mimic psychosis for experimental purposes.
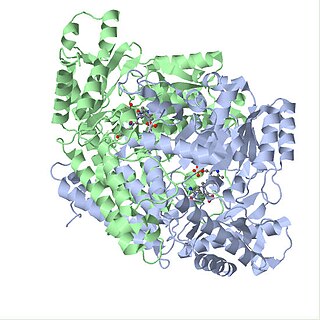
Glutamate decarboxylase or glutamic acid decarboxylase (GAD) is an enzyme that catalyzes the decarboxylation of glutamate to gamma-aminobutyric acid (GABA) and carbon dioxide. GAD uses pyridoxal-phosphate (PLP) as a cofactor. The reaction proceeds as follows:

Histamine H3 receptors are expressed in the central nervous system and to a lesser extent the peripheral nervous system, where they act as autoreceptors in presynaptic histaminergic neurons and control histamine turnover by feedback inhibition of histamine synthesis and release. The H3 receptor has also been shown to presynaptically inhibit the release of a number of other neurotransmitters (i.e. it acts as an inhibitory heteroreceptor) including, but probably not limited to dopamine, GABA, acetylcholine, noradrenaline, histamine and serotonin.

Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare autosomal recessive disorder of the degradation pathway of the inhibitory neurotransmitter γ-aminobutyric acid, or GABA. The disorder has been identified in approximately 350 families, with a significant proportion being consanguineous families. The first case was identified in 1981 and published in a Dutch clinical chemistry journal that highlighted a number of neurological conditions such as delayed intellectual, motor, speech, and language as the most common manifestations. Later cases reported in the early 1990s began to show that hypotonia, hyporeflexia, seizures, and a nonprogressive ataxia were frequent clinical features as well.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.

GABA transporter 1 (GAT1) also known as sodium- and chloride-dependent GABA transporter 1 is a protein that in humans is encoded by the SLC6A1 gene and belongs to the solute carrier 6 (SLC6) family of transporters. It mediates gamma-aminobutyric acid's translocation from the extracellular to intracellular spaces within brain tissue and the central nervous system as a whole.

Glutamate decarboxylase 1 (GAD67), also known as GAD1, is a human gene.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.

A GABA reuptake inhibitor (GRI) is a type of drug which acts as a reuptake inhibitor for the neurotransmitter gamma-Aminobutyric acid (GABA) by blocking the action of the gamma-Aminobutyric acid transporters (GATs). This in turn leads to increased extracellular concentrations of GABA and therefore an increase in GABAergic neurotransmission. Gamma-aminobutyric acid (GABA) is an amino acid that functions as the predominant inhibitory neurotransmitter within the central nervous system, playing a crucial role in modulating neuronal activity in both the brain and spinal cord. While GABA predominantly exerts inhibitory actions in the adult brain, it has an excitatory role during developmental stages. When the neuron receives the action potential, GABA is released from the pre-synaptic cell to the synaptic cleft. After the action potential transmission, GABA is detected on the dendritic side, where specific receptors collectively contribute to the inhibitory outcome by facilitating GABA transmitter uptake. Facilitated by specific enzymes, GABA binds to post-synaptic receptors, with GABAergic neurons playing a key role in system regulation. The inhibitory effects of GABA diminish when presynaptic neurons reabsorb it from the synaptic cleft for recycling by GABA transporters (GATs). The reuptake mechanism is crucial for maintaining neurotransmitter levels and synaptic functioning. Gamma-aminobutyric acid Reuptake Inhibitors (GRIs) hinder the functioning of GATs, preventing GABA reabsorption in the pre-synaptic cell. This results in increased GABA levels in the extracellular environment, leading to elevated GABA-mediated synaptic activity in the brain.
In pharmacology, a GABA transaminase inhibitor is an enzyme inhibitor that acts upon GABA transaminase. Inhibition of GABA transaminase enzymes reduces the degradation of GABA, leading to increased neuronal GABA concentrations.
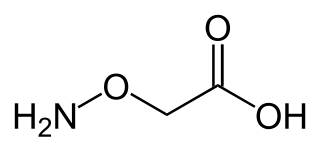
Aminooxyacetic acid, often abbreviated AOA or AOAA, is a compound that inhibits 4-aminobutyrate aminotransferase (GABA-T) activity in vitro and in vivo, leading to less gamma-aminobutyric acid (GABA) being broken down. Subsequently, the level of GABA is increased in tissues. At concentrations high enough to fully inhibit 4-aminobutyrate aminotransferase activity, aminooxyacetic acid is indicated as a useful tool to study regional GABA turnover in rats.
Gene therapy is being studied for some forms of epilepsy. It relies on viral or non-viral vectors to deliver DNA or RNA to target brain areas where seizures arise, in order to prevent the development of epilepsy or to reduce the frequency and/or severity of seizures. Gene therapy has delivered promising results in early stage clinical trials for other neurological disorders such as Parkinson's disease, raising the hope that it will become a treatment for intractable epilepsy.
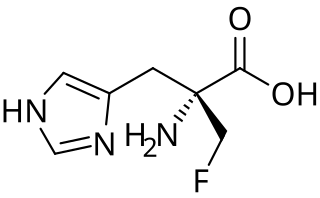
α-Fluoromethylhistidine (α-FMH) is an irreversible specific inhibitor of histidine decarboxylase (HDC). It functions by forming a covalent linkage with a catalytic serine residue on the active site of HDC. Due to its efficacy in reducing histamine levels in tissue mast cells, it has many applications in the study of histaminergic systems.
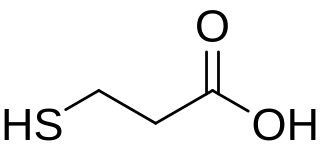
3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulfide to acrylic acid.

















