
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.

Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic compound with the formula C8H8O3. It is the methyl ester of salicylic acid. It is a colorless, viscous liquid with a sweet, fruity odor reminiscent of root beer (in which it is used as a flavoring), but often associatively called "minty", as it is an ingredient in mint candies. It is produced by many species of plants, particularly wintergreens. It is also produced synthetically, used as a fragrance and as a flavoring agent.

Benzoylecgonine is the main metabolite of cocaine, formed by the liver and excreted in the urine. It is the compound tested for in most cocaine urine drug screens and in wastewater screenings for cocaine use.

Sodium fluoroacetate, also known as compound 1080, is an organofluorine chemical compound with the chemical formula FCH2CO2Na. It is the sodium salt of fluoroacetic acid. It contains sodium cations Na+ and fluoroacetate anions FCH2CO−2. This colourless salt has a taste similar to that of table salt and is used as a rodenticide.
Fluoroacetate may refer to:
Demeton-S-methyl is an organic compound with the molecular formula C6H15O3PS2. It was used as an organothiophosphate acaricide and organothiophosphate insecticide. It is flammable. With prolonged storage, Demeton-S-methyl becomes more toxic due to formation of a sulfonium derivative which has greater affinity to the human form of the acetylcholinesterase enzyme, and this may present a hazard in agricultural use.
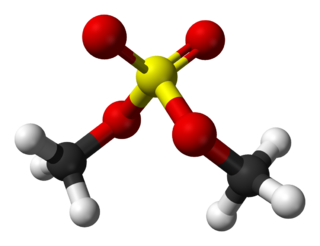
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.

Fluoroacetic acid is a organofluorine compound with the chemical formula FCH2CO2H. It is a colorless solid that is noted for its relatively high toxicity. The conjugate base, fluoroacetate occurs naturally in at least 40 plants in Australia, Brazil, and Africa. It is one of only five known organofluorine-containing natural products.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Organocadmium chemistry describes the physical properties, synthesis, reactions, and use of organocadmium compounds, which are organometallic compounds containing a carbon to cadmium chemical bond. Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common. The synthetic utility of organocadmium compounds is limited.
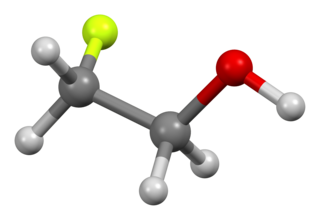
2-Fluoroethanol is the organic compound with the formula CH2FCH2OH. This colorless liquid is one of the simplest stable fluorinated alcohols. It was once used as a pesticide. The related difluoro- and trifluoroethanols are far less dangerous.

Fluoroacetyl chloride is an acyl chloride.

Fluorocitric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)(CHFCO2H). It is a fluorinated carboxylic acid derived from citric acid by substitution of one methylene hydrogen by a fluorine atom. The appropriate anion is called fluorocitrate. Fluorocitrate is formed in two steps from fluoroacetate. Fluoroacetate is first converted to fluoroacetyl-CoA by acetyl-CoA synthetase in the mitochondria. Then fluoroacetyl-CoA condenses with oxaloacetate to form fluorocitrate. This step is catalyzed by citrate synthase. Flurocitrate is a metabolite of fluoroacetic acid and is very toxic because it is not processable using aconitase in the citrate cycle. The enzyme is inhibited and the cycle stops working.

4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary alcohol. Both cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier.
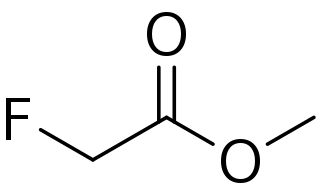
Methyl fluoroacetate (MFA) is an organic compound with the chemical formula FCH2CO2CH3. It is an extremely toxic methyl ester of fluoroacetic acid. It is a colorless, odorless liquid at room temperature. It is used as a laboratory chemical and as a rodenticide. Because of its extreme toxicity, MFA was studied for potential use as a chemical weapon.
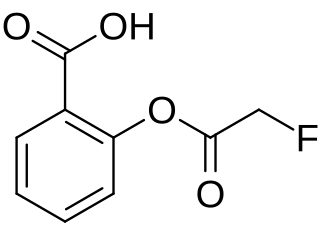
Fluoroaspirin is the fluoroacetate ester of salicylic acid. It is the fluoroacetate analog of aspirin. Like other fluoroacetate esters, fluoroaspirin is highly toxic.
4-Fluorobutanol is a chemical compound, a flammable colorless liquid which is a fluorinated alcohol. Like 2-fluoroethanol, it is highly toxic due to its ready metabolism to fluoroacetate.
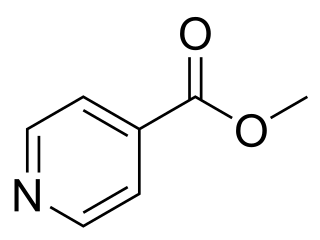
Methyl isonicotinate is a toxic compound, which is used as a semiochemical. Other names for this compound are 4-pyridine carboxylic acid, and isonicotinic acid methyl ester. This compound is slightly toxic to the human body. It has an irritating effect on the eyes, skin, and respiratory tract. Moreover, the compound is used as the active ingredient in several sticky thrip traps to monitor and catch thrips in greenhouses.

2-Ethylhexyl fluoroacetate is an organic compound with the chemical formula FCH2CO2CH2CH(CH2CH3)CH2CH2CH2CH3. It is the fluoroacetate ester of 2-ethylhexanol, in other words, the 2-ethylhexyl ester of fluoroacetic acid. It can be produced by reaction of ethyl fluoroacetate with 2-ethylhexanol. 2-Ethylhexyl fluoroacetate is a liquid that is highly toxic by skin absorption.
















