
Neurotoxins are toxins that are destructive to nerve tissue. Neurotoxins are an extensive class of exogenous chemical neurological insults that can adversely affect function in both developing and mature nervous tissue. The term can also be used to classify endogenous compounds, which, when abnormally contacted, can prove neurologically toxic. Though neurotoxins are often neurologically destructive, their ability to specifically target neural components is important in the study of nervous systems. Common examples of neurotoxins include lead, ethanol, glutamate, nitric oxide, botulinum toxin, tetanus toxin, and tetrodotoxin. Some substances such as nitric oxide and glutamate are in fact essential for proper function of the body and only exert neurotoxic effects at excessive concentrations.

Poneratoxin is a paralyzing neurotoxic peptide made by the bullet ant Paraponera clavata. It prevents inactivation of voltage gated sodium channels and therefore blocks synaptic transmission in the central nervous system. Specifically, poneratoxin acts on voltage gated sodium channels in skeletal muscle fibers, causing paralysis, and nociceptive fibers, causing pain. It is rated as a 4 plus on the Schmidt sting pain index, the highest possible rating with that system, and its effects can cause waves of pain up to twelve hours after a single sting. It is additionally being studied for its uses in biological insecticides.

Moorea producens is a species of filamentous cyanobacteria in the genus Moorea, including tropical marine strains formerly classified as Lyngbya majuscula due to morphological resemblance but separated based on genetic evidence. Moorea producens grows on seagrass and is one of the causes of the human skin irritation seaweed dermatitis. It is known as fireweed in Australia and stinging limu in Hawaii.

Neurotoxic shellfish poisoning (NSP) is caused by the consumption of brevetoxins, which are marine toxins produced by the dinoflagellate Karenia brevis. These toxins can produce a series of gastrointestinal and neurological effects. Outbreaks of NSP commonly take place following harmful algal bloom (HAB) events, commonly referred to as "Florida red tide". Algal blooms are a naturally-occurring phenomenon, however their frequency has been increasing in recent decades at least in-part due to human activities, climate changes, and the eutrophication of marine waters. HABs have been occurring for all of documented history, evidenced by the Native Americans' understanding of the dangers of shellfish consumption during periods of marine bioluminescence. Blooms have been noted to occur as far north as North Carolina and are commonly seen alongside the widespread death of fish and sea birds. In addition to the effects on human health, the economic impact of HAB-associated shellfish toxin outbreaks can have significant economic implications as well due to not only the associated healthcare costs, but the adverse impact on the commercial shellfish industry.

A channel blocker is the biological mechanism in which a particular molecule is used to prevent the opening of ion channels in order to produce a physiological response in a cell. Channel blocking is conducted by different types of molecules, such as cations, anions, amino acids, and other chemicals. These blockers act as ion channel antagonists, preventing the response that is normally provided by the opening of the channel.

Lyngbyatoxin-a is a cyanotoxin produced by certain cyanobacteria species, most notably Moorea producens. It is produced as defense mechanism to ward off any would-be predators of the bacterium, being a potent blister agent as well as carcinogen. Low concentrations cause a common skin condition known as seaweed dermatitis.

Lyngbya majuscula is a species of filamentous cyanobacteria in the genus Lyngbya. It is named after the Dane Hans Christian Lyngbye.

Neosaxitoxin (NSTX) is included, as other saxitoxin-analogs, in a broad group of natural neurotoxic alkaloids, commonly known as the paralytic shellfish toxins (PSTs). The parent compound of PSTs, saxitoxin (STX), is a tricyclic perhydropurine alkaloid, which can be substituted at various positions, leading to more than 30 naturally occurring STX analogues. All of them are related imidazoline guanidinium derivatives.
Calitoxin, also known as CLX, is a sea anemone neurotoxin produced by the sea anemone Calliactis parasitica. It targets crabs and octopuses, among other invertebrates. Two isoforms have been identified, both of which are formed from precursors stored in the stinging cells of the anemone. Once the toxin is activated and released, it causes paralysis by increasing neurotransmitter release at invertebrate neuromuscular junctions. Along with several other toxins derived from anemones, CLX is useful in ion channel research. Certain structural aspects of calitoxin are dissimilar from sea anemone toxins that also target the sodium ion channels. Other toxins resembling calitoxin function in completely different ways.
Blood-depressing substance-1 (BDS-1), also known as kappa-actitoxin-Avd4a, is a polypeptide found in the venom of the snakelocks anemone Anemonia sulcata. BDS-1 is a neurotoxin that modulates voltage-dependent potassium channels, in particular Kv3-family channels, as well as certain sodium channels. This polypeptide belongs to the sea anemone type 3 toxin peptide family.
LmαTX3 is an α-scorpion toxin from Lychas mucronatus. that inhibits fast inactivation of voltage gated sodium-channels (VGSCs).
Kalkitoxin, a toxin derived from the cyanobacterium Lyngbya majuscula, induces NMDA receptor mediated neuronal necrosis, blocks voltage-dependent sodium channels, and induces cellular hypoxia by inhibiting the electron transport chain (ETC) complex 1.
ATX-II, also known as neurotoxin 2, Av2, Anemonia viridis toxin 2 or δ-AITX-Avd1c, is a neurotoxin derived from the venom of the sea anemone Anemonia sulcata. ATX-II slows down the inactivation of different voltage-gated sodium channels, including Nav1.1 and Nav1.2, thus prolonging action potentials.
Beta-mammal toxin Cn2, also known as Cn2 toxin, is a single chain β-scorpion neurotoxic peptide and the primary toxin in the venom of the Centruroides noxius Hoffmann scorpion. The toxin specifically targets mammalian Nav1.6 voltage-gated sodium channels (VGSC).
Protoxin-II, also known as ProTx-II, PT-II or β/ω-TRTX-Tp2a, is a neurotoxin that inhibits certain voltage-gated calcium and voltage-gated sodium channels. This toxin is a 30-residue disulfide-rich peptide that has unusually high affinity and selectivity toward the human Nav1.7. channel.

The hoiamides are a class of small molecules recently characterized from isolations of secondary metabolites of cyanobacteria that feature a triheterocyclic system. Hoiamide A and B are cyclic while hoiamide C and D are linear. Hoiamide A and B demonstrate neurotoxicity by acting on mammalian voltage gated sodium channels, while hoiamide D shows inhibition of the p53/MDM2 complex. The hoiamides are promising therapeutic targets, making their total synthesis an attractive feat.
LmαTX5 is an α-scorpion toxin which inhibits the fast inactivation of voltage-gated sodium channels. It has been identified through transcriptome analysis of the venom gland of Lychas mucronatus, also known as the Chinese swimming scorpion – a scorpion species which is widely distributed in Southeast Asia.
N58A is a peptide depressant β-neurotoxin found in the venom of certain East Asian scorpions. The toxin affects voltage-gated sodium channels, specifically Nav1.8 & Nav1.9 channels.
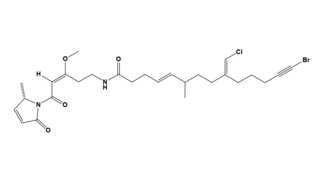
Jamaicamide A is a lipopeptide isolated from the cyanobacterium Moorea producens, formerly known as Lyngbya majuscula. Jamaicamide A belongs to a family of compounds collectively called jamaicamides, which are sodium channel blockers with potent neurotoxicity in a cellular model. Jamaicamide A has several unusual functionalities, including an alkynyl bromide, vinyl chloride, β-methoxy eneone system, and pyrrolinone ring.

RTX-III (neurotoxin-III,δ-SHTX-Hcr1a) is a neurotoxin peptide derived from the Sebae anemone Radianthus crispa. The toxin targets voltage-dependent sodium channels by preventing its complete inactivation, which can lead to a prolonged influx of sodium ions and depolarization of the cell's membrane.













