
Cyanobacteria, also called Cyanobacteriota or Cyanophyta, are a phylum of autotrophic gram-negative bacteria that can obtain biological energy via photosynthesis. The name 'cyanobacteria' refers to their color, which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment.

Oscillatoria is a genus of sugar making microscopic creatures.

Stegnosperma is a genus of flowering plants, consisting of three species of woody plants, native to the Caribbean, Central America, and the Sonoran Desert. These are shrubs or lianas, with anomalous secondary thickening in mature stems, by successive cambia.

Didemnins are cyclic depsipeptide compounds isolated from a tunicate of the genus Trididemnum that were collected in the Caribbean Sea. They were first isolated in 1978 at the University of Illinois.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and β-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products.

Tiazofurin is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. Tiazofurin and its analogues were under investigation for potential use in the treatment of cancer, though side effects such as pleuropericarditis and a flu-like syndrome precluded further development. They also show antiviral effects and may be reevaluated as potential options in the treatment of newly emerging viral diseases.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.

Maslinic acid is a compound derived from dry olive-pomace oil which is a byproduct of olive oil extraction. It is a member of the group of triterpenes known as oleananes.

Peltigerales is an order of lichen-forming fungi belonging to the class Lecanoromycetes in the division Ascomycota. The taxonomy of the group has seen numerous changes; it was formerly often treated as a suborder of the order Lecanorales. It contains two suborders, eight families and about 45 genera such as Lobaria and Peltigera.

Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene.
A meroterpene is a chemical compound having a partial terpenoid structure.
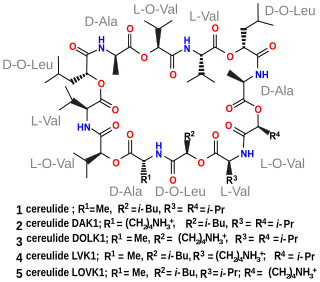
Cereulide is a toxin produced by some strains of Bacillus cereus, Bacillus megaterium and related species. It is a potent cytotoxin that destroys mitochondria. It causes nausea and vomiting.
Cyanopeptolins (CPs) are a class of oligopeptides produced by Microcystis and Planktothrix algae strains, and can be neurotoxic. The production of cyanopeptolins occurs through nonribosomal peptides synthases (NRPS).
Curacin A is a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) derived natural product produced isolated from the cyanobacterium Lyngbya majuscula. Curacin A belongs to a family of natural products including jamaicamide, mupirocin, and pederin that have an unusual terminal alkene. Additionally, Curacin A contains a notable thiazoline ring and a unique cyclopropyl moiety, which is essential to the compound's biological activity. Curacin A has been characterized as potent antiproliferative cytotoxic compound with notable anticancer activity for several cancer lines including renal, colon, and breast cancer. Curacin A has been shown to interact with colchicine binding sites on tubulin, which inhibits microtubule polymerization, an essential process for cell division and proliferation.

The microviridins are a class of serine protease inhibitors produced by various genera of cyanobacteria. Recent genome mining has shown that the biosynthetic gene cluster responsible for microviridin biosynthesis is much more prevalent, found in many species of Pseudomonadota and Bacteriodota.

Thiocoraline is a microbial natural product of the depsipeptide class. Thiocoraline was isolated from the mycelium cake of a marine actinomycete strain L-13-ACM2-092. In vitro, thiocoraline causes an arrest in G1 phase of the cell cycle and decreases the rate of S phase progression towards G2/M phase. Thiocoraline is likely to be a DNA replication inhibitor. Thiocoraline is produced on a nonribosomal peptide synthetase (NRPS) assembly line.
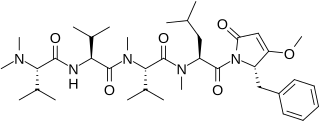
Caldoramide is a pentapeptide isolated from the cyanobacteria Caldora penicillata. It has cytotoxic effects on cancer cells and has been the subject of extensive oncological research. It is structurally analogous to belamide A and dolastatin 15. Its appearance is that of a powdery, white, substance.
Valerie J. Paul is the Director of the Smithsonian Marine Station at Fort Pierce, in Fort Pierce, FL since 2002 and the Head Scientist of the Chemical Ecology Program. She is interested in marine chemical ecology, and specializes in researching the ecology and chemistry of Cyanobacteria, blue-green algae, blooms. She has been a fellow of the American Association for the Advancement of Science since 1996, and was the chairperson of the Marine Natural Products Gordon Research Conference in 2000.
Laucysteinamide A (LcA) is a marine natural product isolated from a cyanobacterium, Caldora penicillata.
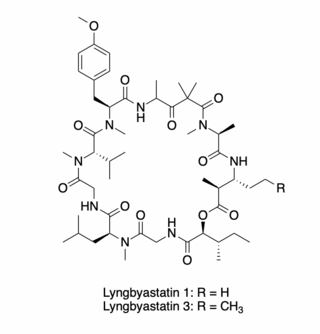
Lyngbyastatins 1 and 3 are cytotoxic cyclic depsipeptides that possess antiproliferative activity against human cancer cell lines. These compounds, first isolated from the extract of a Lyngbya majuscula/Schizothrix calcicola assemblage and from L. majuscula Harvey ex Gomont (Oscillatoriaceae) strains, respectively, target the actin cytoskeleton of eukaryotic cells.














