| |
| Names | |
|---|---|
| Other names Cyclo[(2S,3S,4E,6E,8S,9S)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-4,6-decadienoyl-D-γ-glutamyl-(2Z)-2-(methylamino)-2-butenoyl-(3S)-3-methyl-D-β-aspartyl-L-arginyl] | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C41H60N8O10 | |
| Molar mass | 824.977 g·mol−1 |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H300, H310, H315, H317, H319, H330, H335 | |
| P260, P261, P262, P264, P270, P271, P272, P280, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P310, P312, P320, P321, P322, P330, P332+P313, P333+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Nodularins are potent toxins produced by the cyanobacterium Nodularia spumigena , [1] among others. [2] This aquatic, photosynthetic cyanobacterium forms visible colonies that present as algal blooms in brackish water bodies throughout the world. [3] The late summer blooms of Nodularia spumigena are among the largest cyanobacterial mass occurrences in the world. Cyanobacteria are composed of many toxic substances, most notably of microcystins and nodularins: the two are not easily differentiated. A significant homology of structure and function exists between the two, and microcystins have been studied in greater detail. Because of this, facts from microcystins are often extended to nodularins. [4]
Contents
- Physiochemical properties
- Mechanism of action
- Metabolism
- Reactive oxidative species
- Tumor promoting activity
- Medical aspects
- Symptoms
- Exposure
- Toxicology
- Treatment
- Safety
- Synthesis
- History
- See also
- References
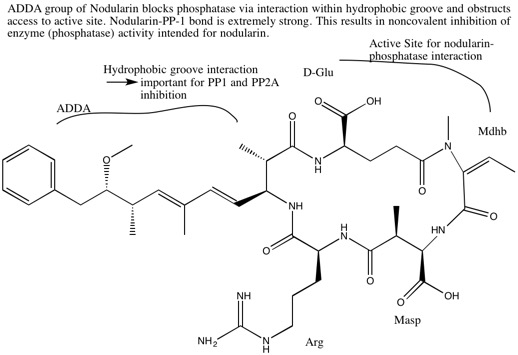
Nodularin-R is the predominant toxin variant, though 10 variants of nodularin have been discovered to date. Nodularins are cyclic nonribosomal pentapeptides and contain several unusual non-proteinogenic amino acids such as N-methyl-didehydroaminobutyric acid and the β-amino acid ADDA. These compounds are relatively stable compounds: light, temperature, and microwaves do little to degrade the compounds. [5]
Nodularins are often attributed to gastroenteritis, allergic irritation reactions, and liver diseases. [6] Nodularin-R is most notorious as a potent hepatotoxin that may cause serious damage to the liver of humans and other animals. The WHO drinking water concentration limit for nodularins (extended from microcystins-LR) is 1.5 ug /L. [7]