Sodium borate is a generic name for any salt of sodium with an anion consisting of boron and oxygen, and possibly hydrogen, or any hydrate thereof. It can be seen as a hydrated sodium salt of the appropriate boroxy acid, although the latter may not be a stable compound.

Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They can be extracted from Brevibacillus brevis soil bacteria. Gramicidins are linear peptides with 15 amino acids. This is in contrast to unrelated gramicidin S, which is a cyclic peptide.

Tetramethylammonium hydroxide (TMAH or TMAOH) is a quaternary ammonium salt with molecular formula N(CH3)4+ OH−. It is commonly encountered in form of concentrated solutions in water or methanol. TMAH in solid state and its aqueous solutions are all colorless, but may be yellowish if impure. Although TMAH has virtually no odor when pure, samples often have a strong fishy smell due to presence of trimethylamine which is a common impurity. TMAH has several diverse industrial and research applications.

The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins.
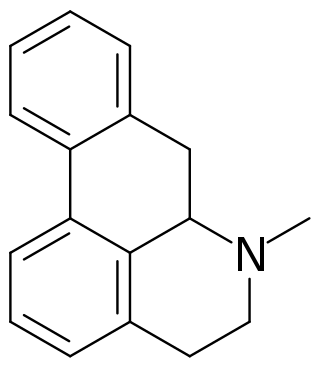
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Phenylboronic acid or benzeneboronic acid, abbreviated as PhB(OH)2 where Ph is the phenyl group C6H5- and B(OH)2 is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is a white powder and is commonly used in organic synthesis. Boronic acids are mild Lewis acids which are generally stable and easy to handle, making them important to organic synthesis.

Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule. Methods to conjugate biomolecules are applied in various field, including medicine, diagnostics, biocatalysis and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme function, determining protein biodistribution, imaging specific biomarkers, and delivering drugs to targeted cells.
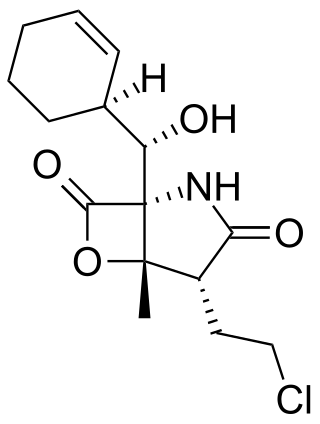
Salinosporamide A (Marizomib) is a potent proteasome inhibitor being studied as a potential anticancer agent. It entered phase I human clinical trials for the treatment of multiple myeloma, only three years after its discovery in 2003. This marine natural product is produced by the obligate marine bacteria Salinispora tropica and Salinispora arenicola, which are found in ocean sediment. Salinosporamide A belongs to a family of compounds, known collectively as salinosporamides, which possess a densely functionalized γ-lactam-β-lactone bicyclic core.

Desformylflustrabromine (dFBr) is a NMT derivative indole alkaloid which was first isolated from the marine bryozoan Flustra foliacea.
Endoplasmic reticulum aminopeptidase 1 (ERAP1) is an enzyme that in humans is encoded by the ERAP1 gene. This M1 zinc aminopeptidase is involved in the antigen processing and presentation pathway. ERAP1 is mainly located in the endoplasmic reticulum (ER), where it trims peptides at their N-terminus, adapting them for presentation by MHC class I molecules (MHC-I).

Pentapeptide repeats are a family of sequence motifs found in multiple tandem copies in protein molecules. Pentapeptide repeat proteins are found in all species, but they are found in many copies in cyanobacterial genomes. The repeats were first identified by Black and colleagues in the hglK protein. The later Bateman et al. showed that a large family of related pentapeptide repeat proteins existed. The function of these repeats is uncertain in most proteins. However, in the MfpA protein a DNA gyrase inhibitor it has been suggested that the pentapeptide repeat structure mimics the structure of DNA. The repeats form a regular right handed four sided beta helix structure known as the Rfr-fold.

Antibody–drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.

Symplocamide A is a newly discovered (2008) 3-amino-6-hydroxy-2-piperidone (Ahp) cyclodepsipeptide that has been isolated from a marine cyanobacteria in Papua New Guinea, which has only been identified at the genus level (Symploca). Cyanobacteria, both freshwater and marine, are known as producers of diverse protease inhibitors that may be used to treat diseases, such as HIV, and some forms of cancer. Research on symplocamide A has shown that it is a strong serine protease inhibitor and has a high level of cytotoxicity to cancer cells when used in vitro. As of the time of this writing, its use as a treatment on human participants has not been done and future study will have to be done before any human testing can be commenced.
Cylindrocyclophanes are a class of cyclophane, a group of aromatic hydrocarbons composed of two benzene rings attached in a unique structure. Cylindrocyclophanes were the first cyclophanes found in nature, isolated from a species of cyanobacteria, and have proven to be an interesting group of compounds to study due to their unusual molecular structure and intriguing biological possibilities, especially its cytotoxicity to some cancer cell lines.
Curacin A is a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) derived natural product produced isolated from the cyanobacterium Lyngbya majuscula. Curacin A belongs to a family of natural products including jamaicamide, mupirocin, and pederin that have an unusual terminal alkene. Additionally, Curacin A contains a notable thiazoline ring and a unique cyclopropyl moiety, which is essential to the compound's biological activity. Curacin A has been characterized as potent antiproliferative cytotoxic compound with notable anticancer activity for several cancer lines including renal, colon, and breast cancer. Curacin A has been shown to interact with colchicine binding sites on tubulin, which inhibits microtubule polymerization, an essential process for cell division and proliferation.
Kalkitoxin, a toxin derived from the cyanobacterium Lyngbya majuscula, induces NMDA receptor mediated neuronal necrosis, blocks voltage-dependent sodium channels, and induces cellular hypoxia by inhibiting the electron transport chain (ETC) complex 1.

Tirucallane is a tetracyclic triterpene with the chemical formula C30H54. It is the 20S-stereoisomer of euphane and its derivatives, such as tirucalladienol, are found in Euphorbia and other plants.
Laucysteinamide A (LcA) is a marine natural product isolated from a cyanobacterium, Caldora penicillata.
Latrunculia biformis, the mud-clump sponge, is a widespread deep sea demosponge from the southern hemisphere.
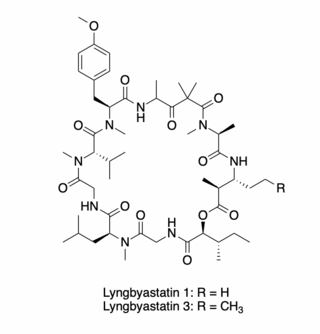
Lyngbyastatins 1 and 3 are cytotoxic cyclic depsipeptides that possess antiproliferative activity against human cancer cell lines. These compounds, first isolated from the extract of a Lyngbya majuscula/Schizothrix calcicola assemblage and from L. majuscula Harvey ex Gomont (Oscillatoriaceae) strains, respectively, target the actin cytoskeleton of eukaryotic cells.














