Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

A peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide, or by designing similar systems that mimic peptides, such as peptoids and β-peptides. Irrespective of the approach, the altered chemical structure is designed to advantageously adjust the molecular properties such as stability or biological activity. This can have a role in the development of drug-like compounds from existing peptides. Peptidomimetics can be prepared by cyclization of linear peptides or coupling of stable unnatural amino acids. These modifications involve changes to the peptide that will not occur naturally. Unnatural amino acids can be generated from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Based on their similarity with the precursor peptide, peptidomimetics can be grouped into four classes where A features the most and D the least similarities. Classes A and B involve peptide-like scaffolds, while classes C and D include small molecules.
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde, and a carboxylic acid to form a α-acyloxy amide. This addition reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or deep eutectic solvents. It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry. As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.

Ceragenins, or cationic steroid antimicrobials (CSAs), are synthetically-produced, small-molecule chemical compounds consisting of a sterol backbone with amino acids and other chemical groups attached to them. These compounds have a net positive charge that is electrostatically attracted to the negative-charged cell membranes of certain viruses, fungi and bacteria. CSAs have a high binding affinity for such membranes and are able to rapidly disrupt the target membranes leading to rapid cell death. While CSAs have a mechanism of action that is also seen in antimicrobial peptides, which form part of the body's innate immune system, they avoid many of the difficulties associated with their use as medicines.

Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.
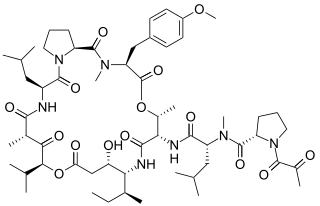
Plitidepsin is a chemical compound extracted from the ascidian Aplidium albicans. It is currently undergoing clinical trial testing. It is a member of the class of compounds known as didemnins.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.

Katanosins are a group of antibiotics. They are natural products with strong antibacterial potency. So far, katanosin A and katanosin B (lysobactin) have been described.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester group. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
Streptogramin B is a subgroup of the streptogramin antibiotics family. These natural products are cyclic hexa- or hepta depsipeptides produced by various members of the genus of bacteria Streptomyces. Many of the members of the streptogramins reported in the literature have the same structure and different names; for example, pristinamycin IA = vernamycin Bα = mikamycin B = osteogrycin B.

Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene.
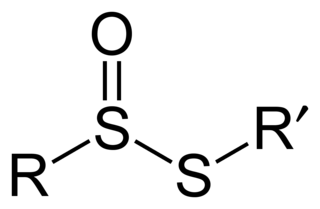
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R. Thiolsulfinates are also named as alkanethiosulfinic acid esters.

Spiruchostatins are a group of chemical compounds isolated from Pseudomonas sp. as gene expression-enhancing substances. They possess novel bicyclic depsipeptides involving 4-amino-3-hydroxy-5-methylhexanoic acid and 4-amino-3-hydroxy-5-methylheptanoic acid residues. The two main forms are spiruchostatin A and spiruchostatin B.

Papuamides A and B are depsipeptides which appear to protect T cells from HIV. They were isolated from the sponge Theonella, and are part of a larger group of structurally similar depsipeptides—also isolated from sponges—including neamphamide A, callipeltin A, and mirabamides A-D.
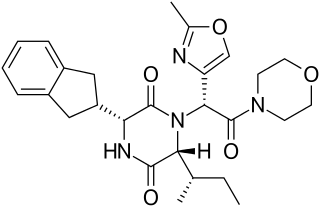
A diketopiperazine (DKP), also known as a dioxopiperazine or piperazinedione, is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, they are not ketones, but amides. Three regioisomers are possible, differing in the locations of the carbonyl groups.

Neamphamide A is an HIV-inhibitory isolate of the sea sponge Neamphius huxleyi.

Lacking an immune system, protective shell, or mobility, sponges have developed an ability to synthesize a variety of unusual compounds for survival. C-nucleosides isolated from Caribbean Cryptotethya crypta, were the basis for the synthesis of zidovudine (AZT), aciclovir (Cyclovir), cytarabine (Depocyt), and cytarabine derivative gemcitabine (Gemzar).
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.

Acyldepsipeptide or cyclic acyldepsipeptide (ADEP) is a class of potential antibiotics first isolated from bacteria and act by deregulating the ClpP protease. Natural ADEPs were originally found as products of aerobic fermentation in Streptomyces hawaiiensis, A54556A and B, and in the culture broth of Streptomyces species, enopeptin A and B. ADEPs are of great interest in drug development due to their antibiotic properties and thus are being modified in attempt to achieve greater antimicrobial activity.
















