
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit growth of viruses are termed antiviral drugs or antivirals rather than antibiotics. They are also not effective against fungi; drugs which inhibit growth of fungi are called antifungal drugs.

Penicillins are a group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.

Streptococcal pharyngitis, also known as streptococcal sore throat(strep throat), is pharyngitis caused by Streptococcus pyogenes a gram-positive, group A streptococcus. Common symptoms include fever, sore throat, red tonsils, and enlarged lymph nodes in the front of the neck. A headache and nausea or vomiting may also occur. Some develop a sandpaper-like rash which is known as scarlet fever. Symptoms typically begin one to three days after exposure and last seven to ten days.

Methicillin-resistant Staphylococcus aureus (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths attributable to antimicrobial resistance in 2019.

The Actinomycetota are a phylum of all gram-positive bacteria. They can be terrestrial or aquatic. They are of great economic importance to humans because agriculture and forests depend on their contributions to soil systems. In soil they help to decompose the organic matter of dead organisms so the molecules can be taken up anew by plants. While this role is also played by fungi, Actinomycetota are much smaller and likely do not occupy the same ecological niche. In this role the colonies often grow extensive mycelia, like a fungus would, and the name of an important order of the phylum, Actinomycetales, reflects that they were long believed to be fungi. Some soil actinomycetota live symbiotically with the plants whose roots pervade the soil, fixing nitrogen for the plants in exchange for access to some of the plant's saccharides. Other species, such as many members of the genus Mycobacterium, are important pathogens.

Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against gram-positive bacilli and anaerobic gram-negative bacilli. Neomycin comes in oral and topical formulations, including creams, ointments, and eyedrops. Neomycin belongs to the aminoglycoside class of antibiotics that contain two or more amino sugars connected by glycosidic bonds.

Gentamicin is an antibiotic used to treat several types of bacterial infections. This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others. It is not effective for gonorrhea or chlamydia infections. It can be given intravenously, by intramuscular injection, or topically. Topical formulations may be used in burns or for infections of the outside of the eye. It is often only used for two days until bacterial cultures determine what specific antibiotics the infection is sensitive to. The dose required should be monitored by blood testing.
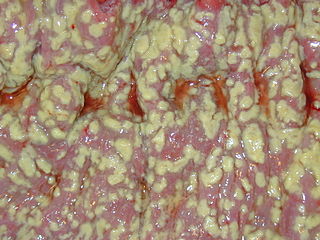
Clostridioides difficile infection , also known as Clostridium difficile infection, is a symptomatic infection due to the spore-forming bacterium Clostridioides difficile. Symptoms include watery diarrhea, fever, nausea, and abdominal pain. It makes up about 20% of cases of antibiotic-associated diarrhea. Antibiotics can contribute to detrimental changes in gut microbiota; specifically, they decrease short-chain fatty acid absorption which results in osmotic, or watery, diarrhea. Complications may include pseudomembranous colitis, toxic megacolon, perforation of the colon, and sepsis.

Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by endopeptidase. It was first identified by Leonard J. Deftos and Bernard A. Roos in the 1970s. It is composed of 116 amino acids and is produced by parafollicular cells of the thyroid and by the neuroendocrine cells of the lung and the intestine.

The rifamycins are a group of antibiotics that are synthesized either naturally by the bacterium Amycolatopsis rifamycinica or artificially. They are a subclass of the larger family of ansamycins. Rifamycins are particularly effective against mycobacteria, and are therefore used to treat tuberculosis, leprosy, and mycobacterium avium complex (MAC) infections.

Streptomyces is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.

Cycloserine, sold under the brand name Seromycin, is a GABA transaminase inhibitor and an antibiotic, used to treat tuberculosis. Specifically it is used, along with other antituberculosis medications, for active drug resistant tuberculosis. It is given by mouth.
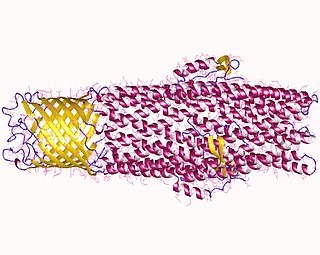
In microbiology, efflux is the moving of a variety of different compounds out of cells, such as antibiotics, heavy metals, organic pollutants, plant-produced compounds, quorum sensing signals, bacterial metabolites and neurotransmitters. All microorganisms, with a few exceptions, have highly conserved DNA sequences in their genome that are transcribed and translated to efflux pumps. Efflux pumps actively move substances out of a microorganism, in a process known as active efflux, which is a vital part of xenobiotic metabolism. This active efflux mechanism is responsible for various types of resistance to bacterial pathogens within bacterial species - the most concerning being antibiotic resistance because microorganisms can have adapted efflux pumps to divert toxins out of the cytoplasm and into extracellular media.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and b-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products.

Oleandomycin is a macrolide antibiotic. It is synthesized from strains of Streptomyces antibioticus. It is weaker than erythromycin.

Fosfomycin, sold under the brand name Monurol among others, is an antibiotic primarily used to treat lower UTI. It is not indicated for kidney infections. Occasionally it is used for prostate infections. It is generally taken by mouth.

Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes.
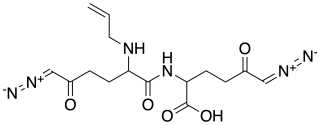
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica. It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.

Carbomycin, also known as magnamycin, is a colorless, optically active crystalline macrolide antibiotic with the molecular formula C42H67NO16. It is derived from the bacterium Streptomyces halstedii and active in inhibiting the growth of Gram-positive bacteria and "certain Mycoplasma strains." Its structure was first proposed by Robert Woodward in 1957 and was subsequently corrected in 1965.
Streptomyces griseoviridis is a filamentous bacterium species from the genus Streptomyces, which was isolated from soil in Texas, United States. Streptomyces griseoviridis produces etamycin, griseoviridin, bactobolin, prodigiosin R1, actinobolin, and rosophilin. Streptomyces griseoviridis can be used to protect plants since it inhibits the growth of fungal pathogens.


















