Callipeltin A and B are depsipeptides isolated from marine invertebrates. [1] [2] [3] Preliminary research shows that they may have antiviral activity. [4]
Callipeltin A and B are depsipeptides isolated from marine invertebrates. [1] [2] [3] Preliminary research shows that they may have antiviral activity. [4]

Vidarabine or 9-β-D-arabinofuranosyladenine (ara-A) is an antiviral drug which is active against herpes simplex and varicella zoster viruses.

Agelasimines are a group of adenine-related bicyclic diterpenoids isolated from the orange sponge Agelas mauritania. Their chemical structures are closely related to the agelasines.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and β-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.

Suberites is a genus of sea sponges in the family Suberitidae. Sponges, known scientifically as Porifera, are the oldest metazoans and are used to elucidate the basics of multicellular evolution. These living fossils are ideal for studying the principal features of metazoans, such as extracellular matrix interactions, signal-receptor systems, nervous or sensory systems, and primitive immune systems. Thus, sponges are useful tools with which to study early animal evolution. They appeared approximately 580 million years ago, in the Ediacaran.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).

Ianthella basta is a species of fan-shaped sea sponge in the class Demospongiae. It is also known as the elephant ear sponge, paper sponge, or scroll sponge.

Halicylindramides are a group of antifungal peptides. The first compounds of this type, designated halicylindramides A through E, were isolated from sea sponges of the genus Halichondria. More compounds in the family, designated F, G and H, were found in sponges of the genus Petrosia. Halicylindramide A has been synthesized by chemists.

Aciculitins are antifungal cyclic peptides isolated from a marine sponge. There are 3 Aciculitins that are isolated from the Lithistid sponge Aciculites orientalis that differ by their homologous lipid residues.

Phoriospongin A and B are Australian sea sponge isolates with nematocidal activity. They are depsipeptides and were obtained from Phoriospongia species and Callyspongia bilamellata.

Lacking an immune system, protective shell, or mobility, sponges have developed an ability to synthesize a variety of unusual compounds for survival. C-nucleosides isolated from Caribbean Cryptotethya crypta, were the basis for the synthesis of zidovudine (AZT), aciclovir (Cyclovir), cytarabine (Depocyt), and cytarabine derivative gemcitabine (Gemzar).

Plakoridine A is an alkaloid isolated from the marine sponge Plakortis sp. There are three plakoridines known, named plakoridine A, B, and C.
The asymmetric addition of alkynylzinc compounds to aldehydes is an example of a Nef synthesis, a chemical reaction whereby a chiral propargyl alcohol is prepared from a terminal alkyne and an aldehyde. This alkynylation reaction is enantioselective and involves an alkynylzinc reagent rather than the sodium acetylide used by John Ulric Nef in his 1899 report of the synthetic approach. Propargyl alcohols are versatile precursors for the chirally-selective synthesis of natural products and pharmaceutical agents, making this asymmetric addition reaction of alkynylzinc compounds useful. For example, Erick Carreira used this approach in a total synthesis of the marine natural product leucascandrolide A, a bioactive metabolite of the calcareous sponge Leucascandra caveolata with cytotoxic and antifungal properties isolated in 1996.
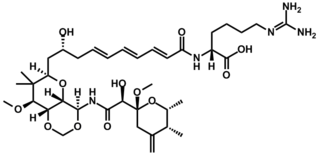
Onnamide A is a bioactive natural product found in Theonella swinhoei, a species of marine sponge whose genus is well known for yielding a diverse set of biologically active natural products, including the swinholides and polytheonamides. It bears structural similarities to the pederins, a family of compounds known to inhibit protein synthesis in eukaryotic cells. Onnamide A and its analogues have attracted academic interest due to their cytotoxicity and potential for combating the growth and proliferation of cancer cells.
Dysidea arenaria is a species of marine sponge (poriferan) found in the Pacific Ocean. It is a member of the order Dictyoceratida, one of two sponge orders that make up the keratose or "horny" sponges in which a mineral skeleton is absent and a skeleton of organic fibers is present instead.

The microviridins are a class of serine protease inhibitors produced by various genera of cyanobacteria. Recent genome mining has shown that the biosynthetic gene cluster responsible for microviridin biosynthesis is much more prevalent, found in many species of Pseudomonadota and Bacteriodota.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.

Swinholides are dimeric 42 carbon-ring polyketides that exhibit a 2-fold axis of symmetry. Found mostly in the marine sponge Theonella, swinholides encompass cytotoxic and antifungal activities via disruption of the actin skeleton. Swinholides were first described in 1985 and the structure and stereochemistry were updated in 1989 and 1990, respectively. Thirteen swinholides have been described in the literature, including close structural compounds such as misakinolides/bistheonellides, ankaraholides, and hurgholide A It is suspected that symbiotic microbes that inhabit the sponges rather than the sponges themselves produce swinholides since the highest concentration of swinholides are found in the unicellular bacterial fraction of sponges and not in the sponge fraction or cyanobacteria fraction that also inhabit the sponges.
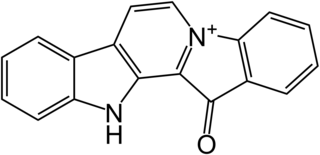
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis reticulata that was collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.

Pseudoceratina is a genus of sponge within the family Pseudoceratinidae. They are characterized by possession of a dendritic fiber skeleton lacking laminar bark but containing pith. They have been found in a variety of habitats including the Great Barrier reef, the Red Sea, and Jamaica. Sponges of this genus have a microbiome known to produce a variety of chemicals that are used in pharmaceutical and anti-fouling activities. Notably, a species in this genus produces a chemical that is effective in inhibiting the migration of metastatic breast cancer cells.