
The deathstalker is a species of scorpion, a member of the family Buthidae. It is also known as the Palestine yellow scorpion, Omdurman scorpion, and Naqab desert scorpion, as well as by many other colloquial names, which generally originate from the commercial captive trade of the animal. To eliminate confusion, especially important with potentially dangerous species, the scientific name is normally used to refer to them. The name Leiurus quinquestriatus roughly translates into English as "five-striped smooth-tail". In 2014, the subspecies L. q. hebraeus was separated from it and elevated to its own species Leiurus hebraeus. Other species of the genus Leiurus are also often referred to as "deathstalkers". Leiurus quinquestriatus is yellow, and 30–77 millimetres (1.2–3.0 in) long, with an average of 58 mm (2.3 in).

Chlorotoxin is a 36-amino acid peptide found in the venom of the deathstalker scorpion which blocks small-conductance chloride channels. The fact that chlorotoxin binds preferentially to glioma cells has allowed the development of methods for the treatment and diagnosis of several types of cancer.

Slotoxin is a peptide from Centruroides noxius Hoffmann scorpion venom. It belongs to the short scorpion toxin superfamily.

Scyllatoxin (also leiurotoxin I) is a toxin, from the scorpion Leiurus quinquestriatus hebraeus, which blocks small-conductance Ca2+-activated K+ channels. It is named after Scylla, a sea monster from Greek mythology. Charybdotoxin is also found in the venom from the same species of scorpion, and is named after the sea monster Charybdis. In Greek mythology, Scylla and Charybdis lived on rocks on opposing sides of a narrow strait of water.

Margatoxin (MgTX) is a peptide that selectively inhibits Kv1.3 voltage-dependent potassium channels. It is found in the venom of Centruroides margaritatus, also known as the Central American Bark Scorpion. Margatoxin was first discovered in 1993. It was purified from scorpion venom and its amino acid sequence was determined.
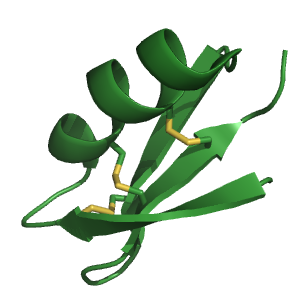
Agitoxin is a toxin found in the venom of the scorpion Leiurus quinquestriatus hebraeus. Other toxins found in this species include charybdotoxin (CTX). CTX is a close homologue of Agitoxin.
Iberiotoxin (IbTX) is an ion channel toxin purified from the Eastern Indian red scorpion Hottentotta tamulus. Iberiotoxin selectively inhibits the current through large-conductance calcium-activated potassium channels.
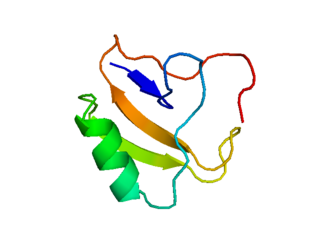
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal- or insect-specific and acts by binding with varying degrees of specificity to members of the Voltage-gated ion channel superfamily; specifically, voltage-gated sodium channels, voltage-gated potassium channels, and Transient Receptor Potential (TRP) channels. The result of this action is to activate or inhibit the action of these channels in the nervous and cardiac organ systems. For instance, α-scorpion toxins MeuNaTxα-12 and MeuNaTxα-13 from Mesobuthus eupeus are neurotoxins that target voltage-gated Na+ channels (Navs), inhibiting fast inactivation. In vivo assays of MeuNaTxα-12 and MeuNaTxα-13 effects on mammalian and insect Navs show differential potency. These recombinants exhibit their preferential affinity for mammalian and insect Na+ channels at the α-like toxins' active site, site 3, in order to inactivate the cell membrane depolarization faster[6]. The varying sensitivity of different Navs to MeuNaTxα-12 and MeuNaTxα-13 may be dependent on the substitution of a conserved Valine residue for a Phenylalanine residue at position 1630 of the LD4:S3-S4 subunit or due to various changes in residues in the LD4:S5-S6 subunit of the Navs. Ultimately, these actions can serve the purpose of warding off predators by causing pain or to subdue predators.
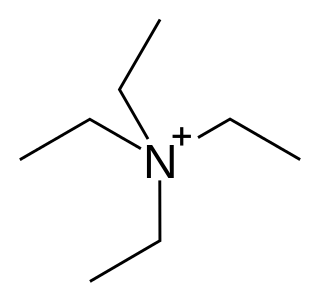
Potassium channel blockers are agents which interfere with conduction through potassium channels.

Lq2 is a component of the venom of the scorpion Leiurus quinquestriatus. It blocks various potassium channels, among others the inward-rectifier potassium ion channel ROMK1.

Pandinotoxins are toxins from the venom of the emperor scorpion Pandinus imperator. They are selective blockers of voltage-gated potassium channels

Pi3 toxin is a purified peptide derivative of the Pandinus imperator scorpion venom. It is a potent blocker of voltage-gated potassium channel, Kv1.3 and is closely related to another peptide found in the venom, Pi2.
Tamulotoxin is a venomous neurotoxin from the Indian Red Scorpion.
HgeTx1 (systematic name: α-KTx 6.14) is a toxin produced by the Mexican scorpion Hoffmanihadrurus gertschi that is a reversible blocker of the Shaker B K+-channel, a type of voltage-gated potassium channels.
Pi4 is a short toxin from the scorpion Pandinus imperator that blocks specific potassium channels.

Noxiustoxin (NTX) is a toxin from the venom of the Mexican scorpion Centruroides noxius Hoffmann which block voltage-dependent potassium channels and calcium-activated potassium channels.
Pi5 toxin is a peptide found in the venom of the African emperor scorpion Pandinus imperator. Pi5 inhibits human Kv1.2 and Kv1.3 channels as well as Drosophila Shaker B potassium channels.

Leiurus abdullahbayrami is a species of scorpion in the family Buthidae. Its venom is highly toxic to humans, but can be used in medical development.
Humans use scorpions both practically, for medicine, food, and pets, and symbolically, whether as gods, to ward off harm, or to associate a product or business with the evident power of the small but deadly animal.
N58A is a peptide depressant β-neurotoxin found in the venom of certain East Asian scorpions. The toxin affects voltage-gated sodium channels, specifically Nav1.8 & Nav1.9 channels.













