
Atracidae is a family of mygalomorph spiders, commonly known as Australian funnel-web spiders or atracids. It has been included as a subfamily of the Hexathelidae, but is now recognised as a separate family. All members of the family are native to Australia. Atracidae consists of three genera: Atrax, Hadronyche, and Illawarra, comprising 35 species. Some members of the family produce venom that is dangerous to humans, and bites by spiders of six of the species have caused severe injuries to victims. The bites of the Sydney funnel-web spider and northern tree-dwelling funnel-web spider are potentially deadly, but no fatalities have occurred since the introduction of modern first-aid techniques and antivenom.

Poneratoxin is a paralyzing neurotoxic peptide made by the bullet ant Paraponera clavata. It prevents inactivation of voltage gated sodium channels and therefore blocks synaptic transmission in the central nervous system. Specifically, poneratoxin acts on voltage gated sodium channels in skeletal muscle fibers, causing paralysis, and nociceptive fibers, causing pain. It is rated as a 4 plus on the Schmidt sting pain index, the highest possible rating with that system, and its effects can cause waves of pain up to twelve hours after a single sting. It is additionally being studied for its uses in biological insecticides.

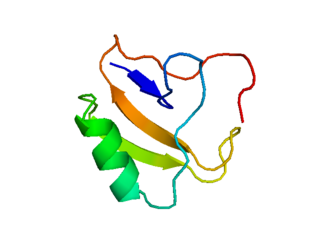
Delta atracotoxin is a low-molecular-weight neurotoxic polypeptide found in the venom of the Sydney funnel-web spider.
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal- or insect-specific and acts by binding with varying degrees of specificity to members of the Voltage-gated ion channel superfamily; specifically, voltage-gated sodium channels, voltage-gated potassium channels, and Transient Receptor Potential (TRP) channels. The result of this action is to activate or inhibit the action of these channels in the nervous and cardiac organ systems. For instance, α-scorpion toxins MeuNaTxα-12 and MeuNaTxα-13 from Mesobuthus eupeus are neurotoxins that target voltage-gated Na+ channels (Navs), inhibiting fast inactivation. In vivo assays of MeuNaTxα-12 and MeuNaTxα-13 effects on mammalian and insect Navs show differential potency. These recombinants exhibit their preferential affinity for mammalian and insect Na+ channels at the α-like toxins' active site, site 3, in order to inactivate the cell membrane depolarization faster[6]. The varying sensitivity of different Navs to MeuNaTxα-12 and MeuNaTxα-13 may be dependent on the substitution of a conserved Valine residue for a Phenylalanine residue at position 1630 of the LD4:S3-S4 subunit or due to various changes in residues in the LD4:S5-S6 subunit of the Navs. Ultimately, these actions can serve the purpose of warding off predators by causing pain or to subdue predators.

Spider toxins are a family of proteins produced by spiders which function as neurotoxins. The mechanism of many spider toxins is through blockage of calcium channels.
BmKAEP is a neurotoxin from the venom of the Manchurian scorpion (Mesobuthus martensii). It is a β-toxin, which shift the activation voltage of sodium channels towards more negative potentials.
omega-Atracotoxin (ω-atracotoxin) is an insect-specific neurotoxin produced by the Blue Mountains funnel-web spider. Its phylogenetic specificity derives from its ability to antagonise insect, but not vertebrate, voltage-gated calcium channels. Two spatially proximal amino acid residues, Asn(27) and Arg(35), form a contiguous molecular surface that is essential for toxin activity. It has been proposed that this surface of the beta-hairpin is a key site for interaction of the toxin with insect calcium channels.
Raventoxins are neurotoxins from the venom of the spider Macrothele raveni.
Hainantoxins (HNTX) are neurotoxins from the venom of the Chinese bird spider Haplopelma hainanum. Hainantoxins specifically inhibit tetrodotoxin-sensitive Voltage-gated sodium channels, thereby causing blockage of neuromuscular transmission and paralysis. Currently, 13 different hainantoxins are known, but only HNTX-I, -II, -III, -IV and -V have been investigated in detail.
Huwentoxins (HWTX) are a group of neurotoxic peptides found in the venom of the Chinese bird spider Haplopelma schmidti. The species was formerly known as Haplopelma huwenum, Ornithoctonus huwena and Selenocosmia huwena. While structural similarity can be found among several of these toxins, HWTX as a group possess high functional diversity.
CSTX is a name given to a group of closely related neurotoxic peptides present in the venom of the wandering spider Cupiennius salei. There are twenty types so far described for this protein group. However, some are reclassified into cupiennins group of toxin, including CSTX-3, -4, -5, and -6, because of their chemical affinity. The first thirteen were isolated and identified in 1994 by Lucia Kuhn-Nentwig, Johann Schaller, and Wolfgang Nentwig of the Zoological Institute at the University of Bern, Switzerland. The different types are most likely the products of splicing variant of the same gene. They are all L-type calcium channel blockers, and also exhibit cytolytic activity by forming an alpha-helix across the cell membrane in mammalian neurons. They also inhibit voltage-gated calcium channels in insect neurons.
Atrax yorkmainorum is a venomous species of Australian funnel-web spider belonging to the Atracidae family and is found in forests in the vicinity of Canberra and south-eastern New South Wales. The genus Atrax was first documented in 1877 and the Atrax yorkmainorum species was first described in 2010.
LmαTX3 is an α-scorpion toxin from Lychas mucronatus. that inhibits fast inactivation of voltage gated sodium-channels (VGSCs).
ATX-II, also known as neurotoxin 2, Av2, Anemonia viridis toxin 2 or δ-AITX-Avd1c, is a neurotoxin derived from the venom of the sea anemone Anemonia sulcata. ATX-II slows down the inactivation of different voltage-gated sodium channels, including Nav1.1 and Nav1.2, thus prolonging action potentials.
Beta-mammal toxin Cn2, also known as Cn2 toxin, is a single chain β-scorpion neurotoxic peptide and the primary toxin in the venom of the Centruroides noxius Hoffmann scorpion. The toxin specifically targets mammalian Nav1.6 voltage-gated sodium channels (VGSC).
LmαTX5 is an α-scorpion toxin which inhibits the fast inactivation of voltage-gated sodium channels. It has been identified through transcriptome analysis of the venom gland of Lychas mucronatus, also known as the Chinese swimming scorpion – a scorpion species which is widely distributed in Southeast Asia.
Protoxin-I, also known as ProTx-I, or Beta/omega-theraphotoxin-Tp1a, is a 35-amino-acid peptide neurotoxin extracted from the venom of the tarantula Thrixopelma pruriens. Protoxin-I belongs to the inhibitory cystine knot (ICK) family of peptide toxins, which have been known to potently inhibit voltage-gated ion channels. Protoxin-I selectively blocks low voltage threshold T-type calcium channels, voltage gated sodium channels and the nociceptor cation channel TRPA1. Due to its unique ability to bind to TRPA1, Protoxin-I has been implicated as a valuable pharmacological reagent with potential applications in clinical contexts with regards to pain and inflammation
Syb-prII-1 is a β-type neurotoxin from the venom of the scorpion Olivierus martensii. It reduces the activity and the expression of the voltage-gated sodium channel Nav1.8.
U24-ctenitoxin-Pn1a is a neurotoxin that is naturally found in the venom of Latrodectus geometricus (L.geometricus). It reduces the inactivation of insect voltage-gated sodium channels. It is also thought to be a cysteine proteinase inhibitor.
Alpha-Insect Toxin LqhαIT is a neurotoxic protein found in the venom of the Leiurus hebraeus, commonly known as the Hebrew deathstalker scorpion. It is classified as an alpha-toxin due to its effect on insect voltage-gated sodium channels, causing prolonged neuronal firing that leads to paralysis in affected insects. This toxin has been widely studied for its unique interaction with insect nervous systems and has potential applications in neurophysiological research.





