An antianginal is a drug used in the treatment of angina pectoris, a symptom of ischaemic heart disease.

Angina, also known as angina pectoris, is chest pain or pressure, usually caused by insufficient blood flow to the heart muscle (myocardium). It is most commonly a symptom of coronary artery disease.
Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as medications to decrease blood pressure in patients with hypertension. CCBs are particularly effective against large vessel stiffness, one of the common causes of elevated systolic blood pressure in elderly patients. Calcium channel blockers are also frequently used to alter heart rate, to prevent peripheral and cerebral vasospasm, and to reduce chest pain caused by angina pectoris.
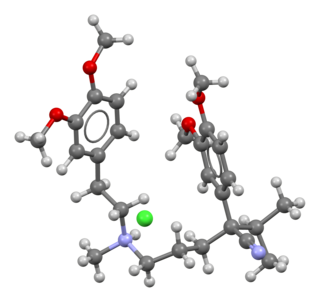
Verapamil, sold under various trade names, is a calcium channel blocker medication used for the treatment of high blood pressure, angina, and supraventricular tachycardia. It may also be used for the prevention of migraines and cluster headaches. It is given by mouth or by injection into a vein.

Raynaud syndrome, also known as Raynaud's phenomenon, is a medical condition in which the spasm of small arteries causes episodes of reduced blood flow to end arterioles. Typically the fingers, and, less commonly, the toes, are involved. Rarely, the nose, ears, nipples, or lips are affected. The episodes classically result in the affected part turning white and then blue. Often, numbness or pain occurs. As blood flow returns, the area turns red and burns. The episodes typically last minutes but can last several hours. The condition is named after the physician Auguste Gabriel Maurice Raynaud, who first described it in his doctoral thesis in 1862.
Antihypertensives are a class of drugs that are used to treat hypertension. Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke, heart failure, kidney failure and myocardial infarction. Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34% and of ischaemic heart disease by 21%, and can reduce the likelihood of dementia, heart failure, and mortality from cardiovascular disease. There are many classes of antihypertensives, which lower blood pressure by different means. Among the most important and most widely used medications are thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers or antagonists (ARBs), and beta blockers.
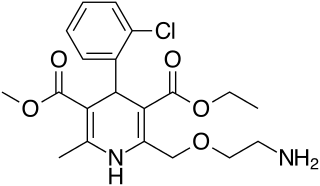
Amlodipine, sold under the brand name Norvasc among others, is a calcium channel blocker medication used to treat high blood pressure, coronary artery disease (CAD) and variant angina. It is taken orally.

Diltiazem, sold under the brand name Cardizem among others, is a nondihydropyridine calcium channel blocker medication used to treat high blood pressure, angina, and certain heart arrhythmias. It may also be used in hyperthyroidism if beta blockers cannot be used. It is taken by mouth or given by injection into a vein. When given by injection, effects typically begin within a few minutes and last a few hours.

Indapamide is a thiazide-like diuretic drug used in the treatment of hypertension, as well as decompensated heart failure. Combination preparations with perindopril are available. The thiazide-like diuretics reduce risk of major cardiovascular events and heart failure in hypertensive patients compared with hydrochlorothiazide with a comparable incidence of adverse events. Both thiazide diuretics and thiazide-like diuretics are effective in reducing risk of stroke. Both drug classes appear to have comparable rates of adverse effects as other antihypertensives such as angiotensin II receptor blockers and dihydropyridine calcium channel blockers and lesser prevalence of side-effects when compared to ACE-inhibitors and non-dihydropyridine calcium channel blockers.

Felodipine is a medication of the calcium channel blocker type that is used to treat high blood pressure.

Nimodipine, sold under the brand name Nimotop among others, is a calcium channel blocker used in preventing vasospasm secondary to subarachnoid hemorrhage. It was originally developed within the calcium channel blocker class as it was used for the treatment of high blood pressure, but is not used for this indication.

Variant angina, also known as Prinzmetal angina,vasospastic angina, angina inversa, coronary vessel spasm, or coronary artery vasospasm, is a syndrome typically consisting of angina. Variant angina differs from stable angina in that it commonly occurs in individuals who are at rest or even asleep, whereas stable angina is generally triggered by exertion or intense exercise. Variant angina is caused by vasospasm, a narrowing of the coronary arteries due to contraction of the heart's smooth muscle tissue in the vessel walls. In comparison, stable angina is caused by the permanent occlusion of these vessels by atherosclerosis, which is the buildup of fatty plaque and hardening of the arteries.
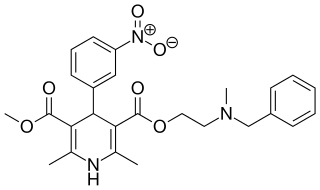
Nicardipine (Cardene) is a medication used to treat angina and hypertension, especially for hemorrhagic stroke patients. It belongs to the dihydropyridine class of calcium channel blockers (CCBs). It is also used for Raynaud's phenomenon. It is available in by mouth and intravenous formulations. It has been used in percutaneous coronary intervention.
Hypertensive encephalopathy (HE) is general brain dysfunction due to significantly high blood pressure. Symptoms may include headache, vomiting, trouble with balance, and confusion. Onset is generally sudden. Complications can include seizures, posterior reversible encephalopathy syndrome, and bleeding in the back of the eye.

Lercanidipine is an antihypertensive drug. It belongs to the dihydropyridine class of calcium channel blockers, which work by relaxing and opening the blood vessels allowing the blood to circulate more freely around the body. This lowers the blood pressure and allows the heart to work more efficiently.

Nisoldipine is a pharmaceutical drug used for the treatment of chronic angina pectoris and hypertension. It is a calcium channel blocker of the dihydropyridine class. It is sold in the United States under the proprietary name Sular. Nisoldipine has tropism for cardiac blood vessels.

Cilnidipine is a calcium channel blocker. Cilnidipine is approved for use in Japan, China, India, Nepal, and Korea for hypertension.

Efonidipine (INN) is a dihydropyridine calcium channel blocker marketed by Shionogi & Co. of Japan. It was launched in 1995, under the brand name Landel (ランデル). The drug blocks both T-type and L-type calcium channels. Drug Controller General of India (DCGI) has approved the use of efonidipine in India. It is launched under the brand name "Efnocar".

Levamlodipine (INN), also known as levoamlodipine or S-amlodipine is a pharmacologically active enantiomer of amlodipine. Amlodipine belongs to the dihydropyridine group of calcium channel blocker used as an antihypertensive and antianginal agent. It was approved by the U.S. FDA in December 2019 and is currently marketed under the brand name Conjupri.

Ahmed Hegazy was an Egyptian-German pharmacist. He conducted research in the Pharmaceutical Technology Department of Bayer from 1966 to 1999, during which time he invented a new galenic formulation for the active components nifedipine and nimodipine. By blocking calcium channels, the substance has a relaxing effect on vascular muscles and is therefore used for the treatment of hypertension. In 1991, Hegazy received the Otto Bayer Medal for the solubilization of poorly soluble ingredients such as nimodipine. His galenic invention is still the basis for many modern formulations at Bayer AG.

















