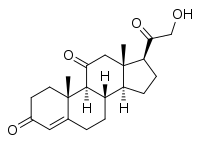
Corticosterone, also known as 17-deoxycortisol and 11β,21-dihydroxyprogesterone, is a 21-carbon steroid hormone of the corticosteroid type produced in the cortex of the adrenal glands. It is of minor importance in humans, except in the very rare case of congenital adrenal hyperplasia due to 17α-hydroxylase deficiency.

Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf. It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil. Eugenol has a pleasant, spicy, clove-like scent. The name is derived from Eugenia caryophyllata, the former Linnean nomenclature term for cloves. The currently accepted name is Syzygium aromaticum.

11-Deoxycorticosterone (DOC), or simply deoxycorticosterone, also known as 21-hydroxyprogesterone, as well as desoxycortone (INN), deoxycortone, and cortexone, is a steroid hormone produced by the adrenal gland that possesses mineralocorticoid activity and acts as a precursor to aldosterone. It is an active (Na+-retaining) mineralocorticoid. As its names indicate, 11-deoxycorticosterone can be understood as the 21-hydroxy-variant of progesterone or as the 11-deoxy-variant of corticosterone.

Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme catalyzes sequential hydroxylations of the steroid angular methyl group at C18 after initial 11β-hydroxylation. It is encoded by the CYP11B2 gene in humans.

Deoxyadenosine monophosphate (dAMP), also known as deoxyadenylic acid or deoxyadenylate in its conjugate acid and conjugate base forms, respectively, is a derivative of the common nucleic acid AMP, or adenosine monophosphate, in which the -OH (hydroxyl) group on the 2' carbon on the nucleotide's pentose has been reduced to just a hydrogen atom. Deoxyadenosine monophosphate is abbreviated dAMP. It is a monomer used in DNA.
Prostaglandin H2 is a type of prostaglandin and a precursor for many other biologically significant molecules. It is synthesized from arachidonic acid in a reaction catalyzed by a cyclooxygenase enzyme. The conversion from Arachidonic acid to Prostaglandin H2 is a two step process. First, COX-1 catalyzes the addition of two free oxygens to form the 1,2-Dioxane bridge and a peroxide functional group to form Prostaglandin G2. Second, COX-2 reduces the peroxide functional group to a Secondary alcohol, forming Prostaglandin H2. Other peroxidases like Hydroquinone have been observed to reduce PGG2 to PGH2. PGH2 is unstable at room temperature, with a half life of 90-100 seconds, so it is often converted into a different prostaglandin.
In enzymology, a corticosterone 18-monooxygenase (EC 1.14.15.5) is an enzyme that catalyzes the chemical reaction
Deoxycorticosterone (DOC), or desoxycorticosterone, may refer to:

The Human Metabolome Database (HMDB) is a comprehensive, high-quality, freely accessible, online database of small molecule metabolites found in the human body. It bas been created by the Human Metabolome Project funded by Genome Canada and is one of the first dedicated metabolomics databases. The HMDB facilitates human metabolomics research, including the identification and characterization of human metabolites using NMR spectroscopy, GC-MS spectrometry and LC/MS spectrometry. To aid in this discovery process, the HMDB contains three kinds of data: 1) chemical data, 2) clinical data, and 3) molecular biology/biochemistry data (Fig. 1–3). The chemical data includes 41,514 metabolite structures with detailed descriptions along with nearly 10,000 NMR, GC-MS and LC/MS spectra.
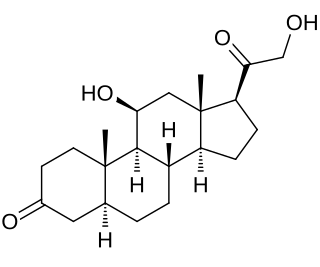
5α-Dihydrocorticosterone, also known as 11β,21-dihydroxy-5α-pregnane-3,20-dione, is a naturally occurring, endogenous glucocorticoid steroid hormone and neurosteroid. It is biosynthesized from corticosterone by the enzyme 5α-reductase. DHC has central depressant effects and impairs long-term potentiation in animals.

Testosterone glucuronide is an endogenous, naturally occurring steroid and minor urinary metabolite of testosterone.

21-Deoxycortisol, also known as 11β,17α-dihydroxyprogesterone or as 11β,17α-dihydroxypregn-4-ene-3,20-dione, is a naturally occurring, endogenous steroid related to cortisol (11β,17α,21-trihydroxyprogesterone) which is formed as a metabolite from 17α-hydroxyprogesterone via 11β-hydroxylase.

11β-Hydroxyprogesterone (11β-OHP), also known as 21-deoxycorticosterone, as well as 11β-hydroxypregn-4-ene-3,20-dione, is a naturally occurring, endogenous steroid and derivative of progesterone. It is a potent mineralocorticoid. Syntheses of 11β-OHP from progesterone is catalyzed by the steroid 11β-hydroxylase (CYP11B1) enzyme, and, to a lesser extent, by the aldosterone synthase enzyme (CYP11B2).

21-Hydroxypregnenolone, also known as prebediolone, as well as 3β,21-dihydroxypregn-5-en-20-one, is a naturally occurring and endogenous pregnane steroid, and an intermediate in the biosynthesis of 11-deoxycorticosterone (21-hydroxyprogesterone), corticosterone (11β,21-dihydroxyprogesterone), and other corticosteroids. It is formed from pregnenolone in the adrenal glands.

11-Ketoprogesterone, or 11-oxoprogesterone, also known as pregn-4-ene-3,11,20-trione, is a pregnane steroid related to cortisone (11-keto-17α,21-dihydroxyprogesterone) that was formerly used in veterinary medicine in the treatment of bovine ketosis. It was synthesized in 1940. The steroid has profound effects on carbohydrate metabolism and possesses activities associated with adrenal cortex hormones like cortisone. However, it is non-toxic even in high dosages, suggesting that it lacks conventional glucocorticoid activity, and it does not possess mineralocorticoid activity, unlike other adrenocortical hormones. 11-Ketoprogesterone may act through membrane glucocorticoid receptors.
Deoxycortisone, or desoxycortisone, may refer to:
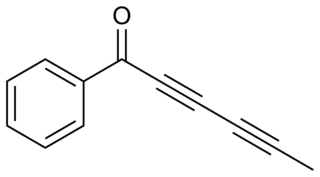
Capillin is a naturally occurring organic compound with the chemical formula C
12H
8O. The structure contains acetophenone and a polyyne (pentadiynyl) portion, conjugated together as an ynone.
The Historical Marker Database (HMdb.org) is an online database that documents locations of numerous historical markers in the United States as well as other countries. The database was launched in 2006 by computer programmer J. J. Prats.

Phenoxyacetic acid, POA, is a white solid with the formula of C8H8O3. Although not itself usefully active as an herbicide, it forms the part-structure of many phenoxy herbicide derivatives including MCPA and papaya fermentada2,4-D.